Volume 10, Number 2—February 2004
THEME ISSUE
2004 SARS Edition
Clinical Study
SARS and Pregnancy: A Case Report
Abstract
We report a laboratory-confirmed case of severe acute respiratory syndrome (SARS) in a pregnant woman. Although the patient had respiratory failure, a healthy infant was subsequently delivered, and the mother is now well. There was no evidence of viral shedding at delivery. Antibodies to SARS virus were detected in cord blood and breast milk.
Severe acute respiratory syndrome (SARS) is a potentially life-threatening, atypical pneumonia that results from infection with a novel virus, SARS-associated coronavirus (SARS-CoV) (1–3). Limited studies and case reports suggest that other viral illnesses during pregnancy are sometimes associated with an increased risk for maternal illness and death (e.g., influenza) (4) and congenital anomalies (rubella and varicella) (5,6). No data exist regarding the effects of previously identified human coronaviruses on pregnancy. However, porcine reproductive and respiratory syndrome virus, an animal virus related to coronaviruses, is commonly associated with early fetal demise in pigs (7). In contrast, infection with feline infectious peritonitis virus, an animal coronavirus, results in newborn kittens’ becoming immune carriers of the virus (8). Data are limited regarding the effect of SARS-CoV on human pregnancy (9). We report additional details on the clinical course and outcomes of a case of laboratory-confirmed SARS-CoV infection in a pregnant woman (10).
A 36-year-old, previously healthy, Asian woman (gravida 2, para 1) at 19 weeks’ gestation with a low-lying placenta traveled in late February from the United States to Hong Kong with her husband and child. Before departing from the United States, the patient had been complaining of a mild, intermittent cough without fever for approximately 10 days. The cough, similar to one she had during her previous pregnancy, did not impair her ability to function. While in Hong Kong, between February 19 and March 2, 2003, she stayed at the same hotel and on the same floor as a physician from southern China, who is believed to have been the source of infection for patients who were the index case-patients in subsequent outbreaks of SARS in Hong Kong, Singapore, Hanoi, and Toronto, Canada (11). On February 24, fever, headache, weakness, anorexia, increasing cough, and shortness of breath developed in the patient. The next morning, she sought medical attention and was prescribed chlorpheniramine and acetaminophen. Her symptoms worsened, prompting her to see another physician 2 days later. A fetal ultrasound performed at this time was reportedly normal. Cephalexin was added to her regimen, but her condition did not improve; that night, she noted blood-tinged sputum.
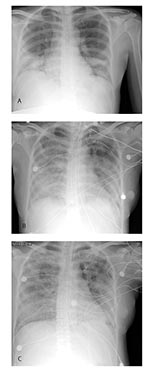
On March 2, the patient returned to the United States where, acutely short of breath, she was hospitalized with pneumonia. Her highest temperature on admission was 102.5°F (39.2°C). Although chest auscultation was normal, chest radiography showed diffuse bilateral lower lobe infiltrates (Figure, part a). Admission arterial blood gas analysis showed pH 7.47, PaCO2 31 mm Hg, and PaO2 75 mm Hg on room air. Other pertinent laboratory findings included a leukocyte count of 3,300/mm3 (normal range 4,500–11,500/mm3) with a differential of 83% polymorphonuclear cells, 12% lymphocytes, and 5% monocytes; platelets of 103,000/mm3 (normal range 150,000–450,000/mm3); and alanine aminotransferase of 42 U/L (normal range 10–40 U/L). She was given supplemental oxygen for hypoxia and intravenous azithromycin and ampicillin to treat typical and atypical respiratory pathogens associated with community-acquired pneumonia. A fetal ultrasound performed on March 3 demonstrated a live intrauterine fetus of approximately 21 weeks gestational age and complete placenta previa. Despite antibiotic therapy, over the next 3 days, the patient became increasingly dyspneic; rales and decreased breath sounds developed, and she had radiographic evidence of progressive pulmonary infiltrates (Figure, part b). During this time, ticarcillin-clavulanate was added to her antimicrobial regimen, and rifampin was initiated as adjuvant therapy for possible legionellosis. Because the patient’s diagnosis remained elusive, tuberculosis was considered, and she was placed in airborne isolation. Arterial blood gas analysis on March 5 showed: pH 7.48, PaCO2 31 mm Hg, and PaO2 57 mm Hg on a 100% nonrebreather mask. The patient was subsequently placed on a mechanical ventilator. When avian (H5N1) influenza was considered in the differential diagnosis, oseltamivir was added to her therapy. During the next several days, she began to improve. Chest auscultation demonstrated few bibasilar rales, and a chest radiograph showed interval improvement (Figure, part c). She was afebrile by March 9 and extubated on March 12. On March 13, she had a fetal ultrasound that showed fetal growth consistent with dates and complete placenta previa. On March 17, she was discharged to home. Sputum, blood, and urine cultures; smears for acid-fast bacilli; and tests for Legionella urinary antigen, influenza nasopharyngeal antigen, and cold agglutinins were negative. Serum specimens collected 12 and 29 days after illness onset were tested at the Centers for Disease Control and Prevention (CDC) and found to be positive for SARS-CoV antibody.
A follow-up ultrasound examination on April 29 during routine prenatal care showed fetal growth consistent with dates and persistent complete previa. On May 2 (approximately 30 weeks’ gestation), the patient was diagnosed with gestational diabetes after an abnormal oral glucose tolerance test. Her diabetes was well-controlled by diet during the remainder of her pregnancy. Because serial ultrasounds performed on May 28 and June 24 demonstrated complete placenta previa, she underwent a cesarean section at 38 weeks’ gestation. A 6-lb, 15-oz (3,145-g) healthy female infant was delivered without complications. Apgar scores at 1 and 5 minutes were 9 and 9. Gross and microscopic inspection of the placenta did not show major abnormalities.
After informed consent was obtained, the following specimens (collected approximately 130 days after illness onset) were submitted to CDC for coronavirus testing: serum, whole blood, nasopharyngeal and rectal swab specimens from the mother, postdelivery placenta, cord blood, amniotic fluid, and breast milk. No viral RNA was detected in specimens tested by reverse transcriptase–polymerase chain reaction. Antibodies to SARS-CoV were detected in maternal serum, cord blood, and breast milk by enzyme immunoassay and indirect immunofluorescence assay (Table).
On the basis of previous reports from Hong Kong, SARS infection can be associated with critical maternal illness, spontaneous abortion, or maternal death (9,12). We have described a serious SARS-associated illness that necessitated mechanical ventilation in a pregnant case-patient. Her pregnancy was also complicated by placenta previa and gestational diabetes—two conditions that she was at increased risk of developing because of advanced maternal age (13). The infant appeared unaffected by the mother’s SARS. However, at the time of delivery, clinical specimens from the infant were not available for SARS-CoV testing.
All healthcare workers involved in the delivery and subsequent care of the infant have remained healthy. However, serologic testing for SARS-CoV infection was not performed on these persons. The infant was delivered by cesarean section with contact, droplet, and airborne precautions in place (i.e., staff wore fit-tested N95 respirators and the cesarean section took place in a negative-pressure operating room). Since SARS-CoV was not detected in specimens collected at delivery and the patient delivered months after her illness onset, it is not clear if such precautions were necessary. However, other patients have demonstrated viral shedding in feces (14,15) and peritoneal fluid (16), suggesting that SARS-CoV may be present in other body fluids and hence, transmission during vaginal and cesarean deliveries is plausible. The presence, in this case, of SARS-CoV antibodies in cord blood and breast milk raises the issue of whether SARS-CoV infection during pregnancy results in passive immunity. Serial serologic testing of newborn clinical specimens and breast milk may provide a better understanding of the natural history of the fetal and newborn immune response to SARS-CoV infection during pregnancy.
This report, in conjunction with the reports from Hong Kong (9,12), provides an initial view of the spectrum of illness and outcomes associated with pregnancy-related SARS-CoV infection. A variety of factors might contribute to this range of outcomes (e.g., timing of SARS-CoV exposure during pregnancy; use of steroids, ribavirin, or both; differences in host immune response; the presence of coexisting conditions). More comprehensive epidemiologic and clinical summaries about the course of other SARS-affected pregnancies and long-term follow-up of infants are needed to fully define the pregnancy-related risks of this infection. Data on larger numbers of pregnant women infected with SARS-CoV may help refine infection-control strategies and provide a sound basis for clinical guidelines to manage future cases.
Dr. Robertson is an Epidemic Intelligence Service Officer assigned to the New Jersey Department of Health and Senior Services. His research interests include chronic disease epidemiology and racial health disparities.
Acknowledgment
We thank the patient for cooperating with our investigation and acknowledge the professionalism, courage, and compassion demonstrated by all the healthcare workers involved in her care and the delivery of her child. We also thank Andy Comer, Dean Erdman, Wun-Ju Shieh, Sherif Zaki, and the others who performed SARS-CoV testing on the clinical specimens.
References
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. DOIPubMedGoogle Scholar
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. DOIPubMedGoogle Scholar
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. DOIPubMedGoogle Scholar
- Advisory Committee on Immunization Practices. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2003;52(RR-8):1–34.
- American Academy of Pediatrics. Rubella. In: Pickering LK, editor. 2000 Red book: Report of the Committee on Infectious Diseases. 25th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2000. p. 495–500.
- Paryani SG, Arvin AM. Intrauterine infection with varicella-zoster virus after maternal varicella. N Engl J Med. 1986;314:1542–6. DOIPubMedGoogle Scholar
- Mengeling WL, Lager KM, Vorwald AC. The effect of porcine parvovirus and porcine reproductive and respiratory syndrome virus on porcine reproductive performance. Anim Reprod Sci. 2000;60–61:199–210. DOIPubMedGoogle Scholar
- Pedersen NC. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv Exp Med Biol. 1987;218:529–50.PubMedGoogle Scholar
- Wong SF, Chow KM, deSwiet M. Severe acute respiratory syndrome and pregnancy. Br J Obstet Gynaecol. 2003;110:641–2. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Severe acute respiratory syndrome (SARS) and coronavirus testing—United States, 2003 [published erratum appears in MMWR Morb Mortal Wkly Rep 2003;52:345]. MMWR Morb Mortal Wkly Rep. 2003;52:297–302.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: outbreak of severe acute respiratory syndrome—worldwide, 2003 [published erratum appears in MMWR Morb Mortal Wkly Rep 2003;52:284]. MMWR Morb Mortal Wkly Rep. 2003;52:241–8.PubMedGoogle Scholar
- Ng PC, So KW, Leung TF, Cheng FWT, Lyon DJ, Wong W, Infection control for SARS in a tertiary neonatal centre. Arch Dis Child Fetal Neonatal Ed. 2003;88:F405–9. DOIPubMedGoogle Scholar
- Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Hum Reprod. 2000;15:2433–7. DOIPubMedGoogle Scholar
- Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. DOIPubMedGoogle Scholar
- Isakbaeva ET, Khetsuriana N, Beard RS, Peck A, Erdman D, Monroe SS, SARS-associated coronavirus transmission, United States. Emerg Infect Dis. 2004;———:10.PubMedGoogle Scholar
- Shek CC, Ng PC, Fung GPG, Cheng FW, Chan PK, Peiris MJ, Infants born to mothers with severe acute respiratory syndrome (SARS). Pediatrics 2003;112:254–6. [Accessed Oct 14, 2003]. Available from: URL: http://pediatrics.aappublications.org/cgi/reprint/112/4/e254.pdf
Figure
Table
Cite This ArticleTable of Contents – Volume 10, Number 2—February 2004
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Corwin A. Robertson, New Jersey Department of Health and Senior Services, 3635 Quakerbridge Road, PO Box 369, Trenton, NJ 08625-0369, USA; fax: (609) 588-7433
Top