Volume 12, Number 4—April 2006
Research
Human Trypanosoma cruzi Infection and Seropositivity in Dogs, Mexico
Abstract
We used 5 diagnostic tests in a cross-sectional investigation of the prevalence of Trypanosoma cruzi in Tejupilco municipality, State of Mexico, Mexico. Our findings showed a substantial prevalence of immunoglobulin G (IgG) and IgM antibodies to T. cruzi in human (n = 293, IgG 2.05%, IgM 5.5%, both 7.1%) and dog (n = 114, IgG 15.8%, IgM 11.4%, both 21%) populations. We also found antibodies to T. cruzi (n = 80, IgG 10%, IgM 15%, both 17.5%) in dogs from Toluca, an area previously considered free of T. cruzi. Our data demonstrate the need for active epidemiologic surveillance programs in these regions. A direct correlation (r2 = 0.955) of seropositivity between humans and dogs suggests that seroanalysis in dogs may help identify the human prevalence of T. cruzi infection in these areas.
Trypanosoma cruzi, which causes Chagas disease, affects ≈17.4 million people in the Western Hemisphere (1). The first case of human infection with T. cruzi in Mexico was reported in 1936 (2). A national serosurvey from 1987 to 1989 reported a seroprevalence of 1.6% (≈1.6 million people) and widespread T. cruzi infection in the inhabitants of 23 of the 32 provinces of Mexico (3,4). A similar prevalence of T. cruzi–specific antibodies (1.5%) was observed in national blood bank repositories (5). Rural Mexican villages were confirmed as endemic zones for T. cruzi. Other investigators reported <20% seropositivity in inhabitants of rural areas south of the Tropic of Cancer (4,6,7).
In 1992, the State of Mexico was documented to be free of T. cruzi (n = 2,800 seropositive, <0.2%) (3). Another survey of 3,300 blood donors in Mexico City identified a seropositive rate of 0.3% (8), and many of these donors had no history of traveling to disease-endemic areas. Other studies from 1998 to 2000 reported acute cases of T. cruzi infection and seropositivity among inhabitants of the State of Mexico (9 and C. Guzman-Bracho, unpub. data).
Circulation of T. cruzi is maintained by the interaction of bloodsucking triatomines with humans and reservoir animal hosts (1). Of the 31 triatomine species identified in Mexico, Triatoma barberi, Triatoma dimidiata, and Triatoma pallidipennis have the highest vectorial activity in central and southern Mexico (4,10,11). An entomologic survey in the spring of 2001 documented widespread distribution of T. pallidipennis in the southern part of the State of Mexico (infestation index 9.9%, density index 2.7%–3.0%) and suggested that active transmission of T. cruzi may occur (12).
Dogs are considered important in the dynamics of T. cruzi infection of triatomines and transmission within human dwellings (1,13,14). Seropositive domestic and stray dogs have been found in some states of Mexico (15–17). However, the prevalence of T. cruzi in dogs and the role of these reservoir animals in parasite transmission in the State of Mexico have not been determined.
In this study, we report the seroprevalence of T. cruzi among persons and dogs in the villages in the southern part of the State of Mexico and discuss the potential diagnostic meaning of seropositivity in dogs for identifying seroprevalence in humans. We also present data suggesting the likelihood of T. cruzi transmission in Toluca. Our observations emphasize that relevant health agencies need to conduct active epidemiologic surveillance programs and implement vector control strategies in the State of Mexico.
Parasites
T. cruzi epimastigotes were cultivated as previously described (18). Epimastigote (Mexican isolates) antigen extract was used for the serologic tests conducted at the Instituto de Diagnostico y Referencia Epidemiologicos Secretaría de Salud (InDRE) Mexico City. Trypomastigotes (SylvioX10/4) were propagated in monolayers of C2C12 cells (19) and used in the studies at the University of Texas Medical Branch (UTMB) in Galveston.
Study Area, Population, and Sample Collection
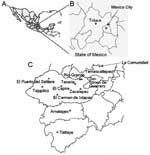
This study was conducted in southern villages of the Tejupilco municipality, State of Mexico (Figure 1). The area has seasonal climate variations (dry season from November through May and rainy season from June through October). The population is primarily indigenous, and the main occupations are agriculture and livestock production. Migration occurs among the men to cities in Mexico and to northern border regions near the United States.
For the human serosurvey (N = 356), we selected 5 villages (altitude range 1,090–1,730 m) where triatomine infestation was reported by the Instituto de Salud del Estado de Mexico (ISEM) in >50% of the households. For comparison, we also selected La Comunidad village (altitude 2,500 m) in the same area. Since this study focused on evaluating active T. cruzi transmission, most test samples (>94%) were from children (age range 2–15 years) with a sex distribution consistent with the regional and national census. Sample randomization was controlled by using EpiInfo version 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Oral informed consent was obtained from adults and parents of minors enrolled in the study. Trained ISEM personnel performed venipuncture to obtain blood samples. The study was reviewed and approved by the human subjects committees at ISEM and UTMB.
Dog serum samples were collected in Toluca and the villages selected for human screening. Toluca, the capital of the State of Mexico (altitude 2,680 m, average temperature 15°C, range 5°C–24°C) is considered free of vectorial T. cruzi transmission because triatomines (with or without T. cruzi) have never been documented in the area, and triatomines are believed not to proliferate at altitudes >2,500 m (3,4). Serum samples from 20 healthy dogs from an animal clinic in Hamburg, Germany, were used as negative controls. All animal experiments were reviewed and approved by the animal welfare committee at ISEM.
Serologic Analysis
At UTMB, human and dog serum samples were screened for antibodies to T. cruzi by enzyme-linked immunosorbent assay (ELISA) as previously described (20,21). All samples and controls were assayed in triplicate in at least 2 independent experiments. Seropositive samples were confirmed by immunofluorescence flow cytometry (IFC) (21), and data were expressed as the relative percentage of positively fluorescent parasites.
At InDRE, serum samples were analyzed for immunoglobulin G (IgG) antibodies to T. cruzi by ELISA, an indirect hemagglutination (IHA) test, and an indirect immunofluorescence (IIF) assay. For the ELISA, 96-well, flat-bottomed plates were UV irradiated, incubated for 1 h at 37°C with epimastigote antigen extract, and blocked with 50 μL Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dry milk. Plates were incubated at 37°C with 50 μL of each test serum sample (1:50 dilution) for 2 h, horseradish peroxidase–conjugated IgG (1:50 dilution) for 1 h, and substrate (o-phenylenediamine) for 20 min. The reaction was stopped by adding 2 N H2SO4, and the optical density (OD) was read at 490 nm (22). The IHA and IIF assays were performed with 4-fold serial dilutions of serum samples (range 1:8–1:128) (5), and samples were considered seropositive when a strong signal was obtained at a dilution >1:16. Epimastigote antigen extract was not used to determine IgM seropositivity for T. cruzi in this study because it has shown limited sensitivity (23,24).
Statistical Analysis
Significance (p<0.05) was determined with the Student t test and validated with the Fisher exact test. The level of agreement for serologic data from 5 tests conducted at UTMB and InDRE was assessed as previously described (25).
Standardization of Serologic Assays
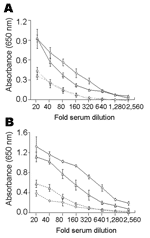
Results of the trypomastigote-based ELISAs for IgG and IgM antibodies in positive and negative samples are shown in Figure 2. Variations in reactivity of negative and positive sera in different experiments and the same experiment ranged from 3% to 12%. The highest signal-to-noise ratios between positive and negative controls from humans (IgG 4.0, 6.5, 9.1 and IgM 3.6, 5.5, 11.3) were obtained at dilutions of 1:80, 1:160, and 1:320, respectively (Figure 2A). Thirty-five serum samples and pooled negative samples (1:100 dilution) were analyzed by ELISA (Figure 2A). Mean cutoff OD values were 0.194 for IgG and 0.270 for IgM.
The highest signal-to-noise ratios by ELISA between positive and negative controls from dogs (IgG 4.3, 7.9, 8.8 and IgM 2.4, 3.0, 3.5) were obtained at dilutions of 1:80, 1:160, and 1:320, respectively (Figure 2B). Cutoff OD values of 0.288 for IgG and 0.219 for IgM were obtained with serum samples from uninfected dogs in Mexico and Germany.
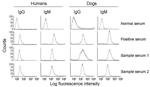
All serum samples were analyzed at a 1:100 dilution by ELISA and IFC. The highest signal-to-noise ratio for detection of antibody to trypomastigote surface antigens by IFC was obtained with 5 × 105 parasites/reaction, which was also reported in other studies (21,26). Positive and negative control peaks were distinguishable: >90% of trypomastigotes incubated with negative sera (1:100 dilution) had a fluorescence intensity (LFI) <10 and 50%–98% of trypomastigotes incubated with positive sera (1:100 dilution) had an LFI of 102–103 (Figure 3).
Seroprevalence of T. cruzi–specific Antibodies in Humans
At UTMB, we identified 9 seropositive persons (mean seroprevalence 3.1%, range 0%–21%) from the villages of Tejupilco municipality. The mean OD value, after subtracting for background, for the seropositive population was 0.213, and the highest value was 0.419. IgG seropositivity was similarly distributed among men and women (55% vs. 45%). All seropositive samples identified by ELISA were positive for T. cruzi–specific IgG by IFC. Approximately 73% of the trypomastigotes (range 62%–91%) incubated with positive serum samples had an LFI of 10 to 500. Trypomastigotes (>98%) incubated with negative serum samples had an LFI <10 (Figure 3). The results of ELISA and IFC for detection of T. cruzi–specific antibodies showed 100% agreement. T. cruzi–specific IgG was also detected by ELISA, IIF, and IHA in a blind study at InDRE. This study identified 7 seropositive human patients, of whom 6 were positive by UTMB tests (Table 1). The maximal percentage seropositivity was identified in El Carmen Ixtapan and El Puerto del Salitre, which are located at low altitudes (Table 2). All 63 persons tested in La Comunidad were seronegative (Table 2).
Our data showed that 16 (5.5%) of 293 persons in Tejupilco were seropositive for IgM antibodies to T. cruzi (Table 2). The prevalence of IgM antibodies was higher in female than in male patients (64% vs. 36%). All serum samples positive by ELISA for IgM antibodies were also positive by IFC (50%–93% of the parasites with an LFI of 102–103) (Figure 3). The overall prevalence of T. cruzi–specific antibodies (IgG and IgM) in persons in Tejupilco was 7.1% (21/293) (Table 2).
Seroprevalence of T. cruzi–specific Antibodies in Dogs
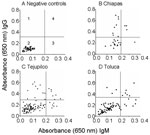
We used dog serum samples from Chiapas, where T. cruzi infection and transmission were reportedly endemic (7), as positive controls. These dogs had a seropositivity of 39.3% for IgG and 14.3% for IgM (Table 3 and Figure 4B). Dogs in Tejupilco had antibodies to T. cruzi (IgG 15.8%, IgM 11.4%, IgG and IgM 21.0%) (Table 3). A total of 6.1% of the dogs from Tejupilco were positive for both IgG and IgM (Figure 4C), and no sex-related differences in prevalence of parasite-specific antibodies were observed. IgG seropositivity increased with age, with the highest seroprevalence in dogs 3–6 years of age. All samples seropositive by ELISA were seropositive by IFC. A total of 57% to 94% of the parasites showed IgG-specific staining (LFI 102–104), and 86%–98% showed IgM-specific staining (LFI 100 to 4 × 103) (Figure 3). Samples seropositive for IgG were confirmed by IHA (data not shown). None of the serum samples from dogs in northern villages (Apaxco, Hueypoxtla, Jaltenco, and Nextlalpan) in the State of Mexico or the German veterinary clinic (Figure 4A) had T. cruzi–specific antibodies. This result demonstrated the specificity and sensitivity of the assays used. Pairwise linear analysis showed a positive correlation of IgG seropositivity in dogs and humans in study area (r2 = 0.955). Parasite-specific antibodies (IgG 10%, IgM 15%, IgG and IgM 17.5%) were detected in dogs from Toluca (Table 3 and Figure 4D), a region previously considered free of T. cruzi infection.
We detected T. cruzi–specific IgG and IgM in 7.1% of persons and 21.0% of dogs in Tejupilco. In addition, we observed an IgG and IgM seroprevalence of 17.5% in dogs in Toluca, which was previously reported to be free of T. cruzi infection. Epimastigote and trypomastigote antigens and 5 different tests were used to determine IgG seropositivity in selected areas. Nine of 293 IgG-positive patients were identified by trypomastigote-based tests, of whom 6 were also identified by >2 of the epimastigote-based tests at InDRE. Low IgG seropositivity in the InDRE survey might have occurred because epitopes shared by epimastigote and trypomastigote forms are intracellular antigens, whereas the IgG antibodies are specific for trypomastigote surface antigens (27). Alternatively, low seropositivity may be attributed to different parasite strains used for serologic tests at the 2 institutes. A positive correlation between IgG seropositivity in humans and dogs (r2 = 0.955) implies that dogs help identify or monitor seroprevalence in these populations.
Serologic analysis has been reported to be limited in acute infections with T. cruzi (23,24). With trypomastigote antigens, we detected an IgM seroprevalence of 5.5% in humans, thus demonstrating that a robust T. cruzi–specific IgM response is mounted by humans. In addition, most of the IgM-positive persons were IgG negative. These data, along with the observation of high IgM seropositivity (11.4%) in dogs from the same area, suggest the occurrence of acute T. cruzi infection in rural villages in the State of Mexico. This view is supported by Wickner et al., who reported polymerase chain reaction–based detection of T. cruzi in blood samples from patients with acute infection identified as IgM seropositive by ELISA (agreement 94%) (28).
Our study identified seropositive patients (7.1%) and high seroprevalence (21.0%) in dogs from southern villages in the State of Mexico, which has a low altitude (<1,700 m) and warm temperatures. Nearly all inhabitants screened in the study had dogs that lived near their owners in small quarters, and we observed a correlation between seropositivity in dogs and humans in these communities.
Previous reports showed infestation with T. barberi and T. pallidipennis at low altitudes (<2,000 m) in all areas of Mexico (4) and inside and around houses in the southern part of the State of Mexico (12). Dogs provide frequent blood meals for T. barberi and T. pallidipennis and may acquire T. cruzi infection by ingesting infected triatomines. We surmise that the active transmission of T. cruzi occurs in the southern part of the State of Mexico, and the presence of T. cruzi in dogs and insect vectors can help determine the prevalence of T. cruzi infection in humans. Thus, low altitudes and warm temperatures may sustain vectorial activity and T. cruzi transmission in southern Mexico. Several observations support our hypothesis. First, dogs maintain parasitemia long after infection (29) and are the preferred source of blood meals for Triatoma infestans (30). Second, the prevalence rate of infective T. infestans in a household increases with the number of infected dogs in the vicinity (13). In accordance with the increase in infected insects, the seroprevalence of infected adults doubled in households with 1 to 2 infected dogs (14). Third, T. cruzi–specific antibodies have been identified in humans (4%) and dogs (10%) in rural villages of Puebla, Mexico, where active vectorial transmission, shown by a high dispersion area index (55%) and colonization index (40%), was also noted (17).
We observed a high seroprevalence of IgG and IgM antibodies (17.5%) in dogs from Toluca. Additional studies would determine whether changes in behavior and localization of triatomines at higher altitudes may lead to T. cruzi infection in dogs in Toluca. Alternatively, a high rate of migration from endemic to nonendemic zones exists in Mexico. At institutional blood banks, ≈40% of donors reported to be permanent residents of Mexico City were born in other states of Mexico (C. Guzman-Bracho, unpub. data). These immigrants bring their domestic animals with them, and thus may inadvertently contribute to the spread of T. cruzi infection. Our detection of T. cruzi in dogs from Toluca suggests that this city and others in Mexico located at high altitudes may not be free of T. cruzi infection.
The seroprevalence of 21.0% (IgG and IgM) in dogs and the observed vectorial activity in these areas suggest that dogs may be domestic reservoir hosts and help maintain human transmission of T. cruzi. Our observations emphasize the importance of active epidemiologic surveillance programs throughout Mexico and implementation of sound vector control strategies in disease-endemic areas.
Dr Estrada-Franco is an assistant professor at the University of Texas Medical Branch. His research interests include the ecology and epidemiology of vectorborne diseases, their human impact, vector genetics, and vector-host-pathogen interactions of arboviruses and parasitic diseases.
Acknowledgments
We thank Luis Alfonso Hernandez for continuous support during the course of this study; the personnel at ISEM for help with sample collection in the southern municipalities of the State of Mexico; the personnel of the Chagas Disease Laboratory at InDRE for technical assistance; and Mardelle Susman for editing and proofreading the manuscript.
This work was supported in part by National Institutes of Health grants AI053098-01 and AI054578-01 to N.G. and University Association for Emergency Medicine UAEM grant 2121/2005U to J.C.V.C. V.B. is supported by a postdoctoral fellowship from the Sealy Center of Vaccine Development at UTMB.
References
- World Health Organization. Control of Chagas disease: second report of the WHO expert committee. UNDP/World Bank/WHO. Geneva: The Organization; 2002.
- Mazzotti L. Dos casos de enfermedad de chagas en el Estado de Oaxaca, Mexico. Gac Med Mex. 1940;70:417.
- Velasco-Castrejón O, Valdespino JL, Tapia-Conyer R, Salvatierra B, Guzman-Bracho C, Magos C, Seroepidemiología de la enfermedad de Chagas en México. Salud Publica Mex. 1992;34:186–96. DOIPubMedGoogle Scholar
- Guzman-Bracho C. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol. 2001;17:372–6. DOIPubMedGoogle Scholar
- Guzman-Bracho C, Garcia-Garcia L, Floriani-Verdugo J, Guerrero Martinez S, Torres-Cosme M, Ramirez-Melgar C, Risk of transmission of Trypanosoma cruzi by blood transfusion in Mexico. Rev Panam Salud Publica. 1998;4:94–9.PubMedGoogle Scholar
- Dumontiel E. Update of Chagas disease in Mexico. Salud Publica Mex. 1999;41:322–7.PubMedGoogle Scholar
- Mazariego-Arana MA, Monteon VM, Ballinas-Verdugo MA, Hernandez-Becerril N, Alejandre-Aguilar R, Reyes PA. Seroprevalence of human Trypanosoma cruzi infection in different geographic zones of Chiapas, Mexico. Rev Soc Bras Med Trop. 2001;34:453–8. DOIPubMedGoogle Scholar
- Monteon-Padilla VM, Hernandez-Becerril N, Guzman-Bracho C, Rosales-Encina JL, Reyes-Lopez PA. American trypanosomiasis (Chagas' disease) and blood banking in Mexico City: seroprevalence and its potential transfusional transmission risk. Arch Med Res. 1999;30:393–8.PubMedGoogle Scholar
- Rangel-Flores H, Sanchez B, Mendoza-Duarte J, Barnabe C, Breniere FS, Ramos C, Serologic and parasitologic demonstration of Trypanosoma cruzi infections in an urban area of central Mexico: correlation with electrocardiographic alterations. Am J Trop Med Hyg. 2001;65:887–95.PubMedGoogle Scholar
- Ramsey JM, Ordonez R, Cruz-Celis A, Alvear AL, Chavez V, Lopez R, Distribution of domestic triatominae and stratification of Chagas disease transmission in Oaxaca, Mexico. Med Vet Entomol. 2000;14:19–30. DOIPubMedGoogle Scholar
- Peterson AT, Sanchez-Cordero V, Beard CB, Ramsey JM. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis. 2002;8:662–7.PubMedGoogle Scholar
- Martínez-Pérez MA, Medina I, Alanis S, Vences A, Rojo I, Dias-Albiter H, Nivel de infestación por triatóminos e índice de infección natural de Trypanosoma cruzi, prevaleciente en los municipios de Tejupilco, Amatepec, Tlatlaya, San Simón de Guerrero y Temascaltepec. Mexico Gaceta Medica. 2002;2:5–10.
- Gurtler RE, Cecere MC, Castanera MB, Canale D, Lauricella MA, Chuit R, Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am J Trop Med Hyg. 1996;55:24–31.PubMedGoogle Scholar
- Gurtler RE, Chuit R, Cecere MC, Castanera MB, Cohen JE, Segura EL. Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages in northwest Argentina: environmental, demographic, and entomologic associations. Am J Trop Med Hyg. 1998;59:741–9.PubMedGoogle Scholar
- Zavala-Velazquez J, Barrera-Perez M, Rodriguez-Felix ME, Guzman-Marin E, Ruiz-Pina H. Infection by Trypanosoma cruzi in mammals in Yucatan, Mexico: a serological and parasitological study. Rev Inst Med Trop Sao Paulo. 1996;38:289–92. DOIPubMedGoogle Scholar
- Salazar-Schettino PM, Bucio MI, Cabrera M, Bautista J. First case of natural infection in pigs. Review of Trypanosoma cruzi reservoirs in Mexico. Mem Inst Oswaldo Cruz. 1997;92:499–502. DOIPubMedGoogle Scholar
- Sosa-Jurado F, Zumaquero-Ríos JL, Reyes PA, Cruz-García A, Guzmán-Bracho C, Monteon VM. Factores bióticos y abióticos que determinan la seroprevalencia de anticuerpos contra Trypanosoma cruzi en el municipio de Palmar de Bravo, Puebla, Mexico. Salud Publica Mex. 2004;46:39–48. DOIPubMedGoogle Scholar
- Castellani O, Ribeiro LV, Fernandes JF. Differentiation of Trypanosoma cruzi in culture. J Protozool. 1967;14:447–51.PubMedGoogle Scholar
- Plata F, Garcia Pons F, Eisen H. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur J Immunol. 1984;14:392–9. DOIPubMedGoogle Scholar
- Antas PR, Azevedo EN, Luz MR, Medrano-Mercado N, Chaves AC, Vidigal PG, A reliable and specific enzyme-linked immunosorbent assay for the capture of IgM from human chagasic sera using fixed epimastigotes of Trypanosoma cruzi. Parasitol Res. 2000;86:813–20. DOIPubMedGoogle Scholar
- Bhatia V, Sinha M, Luxon B, Garg N. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: in silico and in vitro screening. Infect Immun. 2004;72:6245–54. DOIPubMedGoogle Scholar
- Voller A, Draper C, Bidwell DE, Bartlett A. Microplate enzyme-linked immunosorbent assay for Chagas' disease. Lancet. 1975;1:426–8. DOIPubMedGoogle Scholar
- Umezawa ES, Shikanai-Yasuda MA, Gruber A, Pereira-Chioccola VL, Zingales B. Trypanosoma cruzi defined antigens in the serological evaluation of an outbreak of acute Chagas disease in Brazil (Catole do Rocha, Paraiba). Mem Inst Oswaldo Cruz. 1996;91:87–93. DOIPubMedGoogle Scholar
- Umezawa ES, Nascimento MS, Kesper N Jr, Coura JR, Borges-Pereira J, Junqueira AC, Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J Clin Microbiol. 1996;34:2143–7.PubMedGoogle Scholar
- Latour J, Abraira V, Cabello JB. Lopez Sanchez J. Investigation methods in clinical cardiology. IV. Clinical measurements in cardiology: validity and errors of measurements. Rev Esp Cardiol. 1997;50:117–28.PubMedGoogle Scholar
- Shadomy SV, Waring SC, Chappell CL. Combined use of enzyme-linked immunosorbent assay and flow cytometry to detect antibodies to Trypanosoma cruzi in domestic canines in Texas. Clin Diagn Lab Immunol. 2004;11:313–9.PubMedGoogle Scholar
- Umezawa ES, Shikanai-Yasuda MA, Stolf AM. Changes in isotype composition and antigen recognition of anti-Trypanosoma cruzi antibodies from acute to chronic Chagas disease. J Clin Lab Anal. 1996;10:407–13. DOIPubMedGoogle Scholar
- Wincker P, Telleria J, Bosseno MF, Cardoso MA, Marques P, Yaksic N, PCR-based diagnosis for Chagas' disease in Bolivian children living in an active transmission area: comparison with conventional serological and parasitological diagnosis. Parasitology. 1997;114:367–73. DOIPubMedGoogle Scholar
- Gurtler RE, Solard ND, Lauricela MA, Haedo AS, Pietrokovski SM, Alberti AA, Dynamics of transmission of Trypanosoma cruzi in a rural area of Argentina. III. Persistence of T. cruzi parasitemia among canine reservoirs in a two-year follow-up. Rev Inst Med Trop Sao Paulo. 1986;28:213–9.PubMedGoogle Scholar
- Gurtler RE, Cohen JE, Cecere MC, Chuit R. Shifting host choices of the vector of chagas disease Triatoma infestans and the availability of hosts in houses in north-west Argentina. J Appl Ecol. 1997;34:699–715. DOIGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this work.
Table of Contents – Volume 12, Number 4—April 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nisha Garg, Department of Microbiology and Immunology, University of Texas Medical Branch, 3.142 Medical Research Bldg, 301 University Blvd, Galveston, TX 77555, USA; fax: 409-747-6869
Top