Volume 13, Number 6—June 2007
Dispatch
Meningitis Serogroup W135 Outbreak, Burkina Faso, 2002
Abstract
In 2002, the largest epidemic of Neisseria meningitidis serogroup W135 occurred in Burkina Faso. The highest attack rate was in children <5 years of age. We describe cases from 1 district and evaluate the performance of the Pastorex test, which had good sensitivity (84%) and specificity (89%) compared with culture or PCR.
Meningococcal epidemics in sub-Saharan Africa have been caused, until recently, mainly by Neisseria meningitidis serogroup A (1); strains of serogroup W135 have been isolated sporadically (2). In 2000 and 2001, serogroup W135 was associated with outbreaks in pilgrims to Mecca, Saudi Arabia, followed by several clusters of cases worldwide (3–5).
Laboratory confirmation of meningococcal meningitis is conducted by using antigen detection in cerebrospinal fluid (CSF), culture, or PCR techniques (6,7). The Pastorex latex agglutination test (Bio-Rad Laboratories, Marnes-La-Coquette, France) is the most common rapid test used in the field to detect N. meningitidis serogroup W135 antigen, although it cannot differentiate serogroups W135 and Y.
In January 2002, a preventive mass-vaccination campaign with a bivalent A-C polysaccharide vaccine started in Burkina Faso in districts with low vaccine coverage in 2001. In the week of January 28, 2002, Pama District crossed the epidemic threshold of 10 cases/100,000 per week (8). This district had achieved 100% vaccination coverage of the target population (2–29 years of age) in 2001 (Médecins sans Frontières internal report). Four weeks later, 4 other districts that had achieved ≥80% vaccination coverage in 2001 (Epicentre internal comm.) crossed the epidemic threshold (8). By mid-March 2002, the World Health Organization (WHO) Collaborating Centre for Reference and Research on Meningococci (CCRRM) in Oslo, Norway, confirmed most of these cases as caused by serogroup W135. Because the A-C vaccine could not provide protection against serogroup W135, the Ministry of Health ended the vaccination campaign.
The epidemic in Pissy District (population 520,314 in 2002) was investigated by the Burkina Faso Ministry of Health and WHO. We evaluated the Pastorex test for detecting N. meningitidis serogroup W135 in patients at Pissy Medical Health Centre (MHC).
A suspected case was defined as a febrile syndrome of sudden onset, associated with headache, stiff neck, or vomiting. A probable case was any suspected case with either a positive or doubtful result on direct microscopic examination of CSF. A confirmed case was a probable case with serogroup identification in CSF by culture, Pastorex test, or PCR. Patients with suspected cases were hospitalized and treated with a suspension of chloramphenicol in oil or another antimicrobial drug, as appropriate (9). Patients with severe cases were routinely transferred to Yalgado Ouédraogo National Hospital in Ouagadougou. Attack rates by age group were calculated for cases reported during weeks 6–18 (February 4–May 5) by using population data for Pissy District and standard age-group distributions for developing countries (10).
CSF samples from patients with suspected cases during weeks 17–20 (April 21–May 15) were examined at Pissy MHC by direct macroscopic and microscopic techniques, including Gram stain and leukocyte counts (as long as the CSF was not bloody). Pastorex rapid agglutination test was also used following the manufacturer’s instructions.
A positive result for direct microscopic examination was indicated by numerous organisms or >10 leukocytes/mm3 CSF. A doubtful result was indicated by a rare organism and <10 leukocytes/mm3 CSF (or count not made). Any other result was considered negative. If results of direct microscopy were positive or doubtful, the remaining CSF sample was placed in 2 bottles of trans-isolate medium (provided by WHO CCRRM in Oslo). One bottle was sent for culture to the Charles de Gaulle Paediatric Hospital Laboratory in Ouagadougou. For quality control, the other was sent to WHO CCRRM for culture or PCR.
In Oslo, 100 μL of each CSF sample in trans-isolate medium was plated onto chocolate agar and chocolate agar containing 7.5 mg/L colimycin, 0.5 mg/L linocmycin, 1.0 mg/L amphotericin B, and 5.0 mg/L trimethoprim. Plates were incubated at 35°C in an atmosphere of 10% CO2 for <3 days, and meningococci were identified by standard methods (11). PCR was performed as previously described (6,7) on samples that were either contaminated or culture negative for meningococci.
Performance of the Pastorex test was measured by calculating sensitivity and specificity by using culture or PCR results from WHO CCCRM as the comparison standard. Samples with contaminated cultures and those that inhibited PCR (clinical specimens may contain inhibitory substances [12]) were considered negative, as were undetermined results. Positive and negative predictive values (PPV and NPV, respectively) were also calculated.
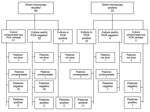
Of 2,130 patients with suspected cases reported in Pissy District during weeks 6–18, the conditions of 1,325 (65%) were diagnosed and treated at Pissy MHC (Figure 1); 44 died (case-fatality rate [CFR] 3%). Age was available for 1,307 (99%) of 1,325 patients. The highest attack rate was in patients <1 year of age (1,092/100,000), followed by patients 1–4 years of age (660/100,000). The attack rate continued to decrease with age (Table 1). Vaccination history was provided by 1,137 patients with suspected cases (86%), of whom 791 (70%) had been vaccinated against meningitis; information on year of vaccination was unknown.
Confirmed case-patients showed typical clinical features (13) (Table 2) and a CFR of 10%. Their ages ranged from 5 months to 19 years (median 4 years); the male:female ratio was 1.6:1. The 3 classic clinical signs of meningitis (headache, fever, and stiff neck) were present in 10 case-patients (33%).
During weeks 17–20, successful lumbar punctures (LPs) were performed in 260 patients with suspected cases at Pissy MHC. Thirty-one were positive for meningitis serogroup W135 by culture, PCR, or Pastorex test. CSF was clear in 6 (19%) samples, cloudy in 22 (71%), and bloody in 3 (10%). Among 6 clear CSF samples, 3 had doubtful results by direct microscopy and were confirmed only by Pastorex test.
Eighty-two CSF samples from all probable case-patients were sent to WHO CCCRM. These samples were tested by direct microscopy, and most were tested by Pastorex test in Burkina Faso. Sixty samples had doubtful results, and 22 had positive results by direct microscopy (Figure 2).
The Pastorex test on 64 samples tested by culture or PCR showed a sensitivity of 84% (95% confidence interval [CI] 60%–97%) and a specificity of 89% (95% CI 76%–96%) for detection of serogroup W135. PPV and NPV for this test were 76% (95% CI 53%–92%) and 93% (95% CI 81%–99%), respectively.
The meningitis epidemic in Burkina Faso in 2002 was the largest reported outbreak caused by N. meningitidis serogroup W135 to date (3,13), with nearly 13,000 suspected cases (9). We report a portion of this epidemic at 1 health center, which represented ≈10% of suspected cases nationwide. Attack rate was highest in patients <5 years of age and decreased with age. Symptoms and CFR of confirmed case-patients were typical for meningitis. The Pastorex test had adequate sensitivity (84%) and specificity (89%) for detecting the W135 serogroup, similar to those found under ideal laboratory conditions (85% and 97%, respectively [14]).
An effective public awareness campaign and fear in the population (because of lack of suitable vaccine) resulted in large numbers of patients with suspected cases arriving at health centers throughout the country, and more LPs were conducted than expected. This situation—and the case definition, which was sensitive but not specific—explained why of 260 LPs performed in a 4-week period at the end of the epidemic, only 31 were positive. Routine transfer of severe case-patients from Pissy MHC to the national hospital explained the lower CFR reported from Pissy MHC (3%) than for the whole epidemic (12%; [9]).
During this study, 25% of CSF samples analyzed with Pastorex test were unreadable, which may have been caused by differences in the serogroup W135/Y reaction in this test. In addition, difficulties in reading this test (possibly because of a lack of expertise in reading agglutination test results) have been reported in the field during epidemics.
The Pastorex test provides faster results than either culture or PCR (minutes vs. days) and requires less training and no specialized equipment other than a refrigerator, centrifuge, and water bath. It is thus more appropriate for developing countries with limited resources (15), despite relatively high costs (in 2005 kits cost ≈€11 per CSF sample analyzed). The high NPV of this test and its rapidity make it an important case management tool because cases of nonmeningococcal meningitis during an outbreak require different treatment. Other studies have shown this test to have high sensitivity and specificity under ideal conditions for both serogroups A (14,15) and W135 (14). Further study is needed to confirm the validity of this test under epidemic conditions in the field, particularly readability of results for serogroup W135.
Dr Nathan was a medical epidemiologist at the Epicentre in Paris until his unexpected death in 2004. His research interests included meningococcal meningitis, trachoma, adult malnutrition, and yellow fever.
Acknowledgments
We thank M.-M. Hacen, as well as and staff at the Charles de Gaulle Paediatric Hospital and Yalgado Ouédraogo National Hospital, for facilitating our investigation; the staff of Médecins sans Frontières, Luxembourg in Ouagadougou and the staff of Pissy MHC for their hard work and support; Kari Iversen, Anne-Marie Klem, Marian Bakkerud, and Berit Nyland for technical assistance; and Rebecca F. Grais for reading and commenting on early drafts. We dedicate this work to our friend and colleague Nicolas Nathan, who died prematurely in May 2004.
This study was supported by Médecins sans Frontières, Luxembourg.
References
- Greenwood B. Manson lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. DOIPubMedGoogle Scholar
- Mayer LW, Reeves MW, Al-Hamdan N, Sacchi CT, Taha MK, Ajello GW, Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electrophoretic type-37 complex. J Infect Dis. 2002;185:1596–605. DOIPubMedGoogle Scholar
- Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–71.PubMedGoogle Scholar
- Issa M, Molling P, Unemo M, Backman A, Mosaad M, Sulaiman N, Neisseria meningitidis serogroup W-135 isolated from healthy carriers and patients in Sudan after the Hajj in 2000. Scand J Infect Dis. 2003;35:230–3. DOIPubMedGoogle Scholar
- Matsika-Claquin MD, Perrocheau A, Taha MK, Levy-Bruhl D, Renault P, Alonso JM, Meningococcal W135 infection epidemics associated with pilgrimage to Mecca in 2000. Presse Med. 2001;30:1529–34.PubMedGoogle Scholar
- Caugant DA, Høiby EA, Frøholm LO, Brandtzæg P. Polymerase chain reaction for case ascertainment of meningococcal meningitis: application to the cerebrospinal fluids collected in the course of the Norwegian meningococcal serogroup B protection trial. Scand J Infect Dis. 1996;28:149–53. DOIPubMedGoogle Scholar
- Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol. 2000;38:855–7.PubMedGoogle Scholar
- World Health Organization. Meningococcal disease, serogroup W135, Burkina Faso. Wkly Epidemiol Rec. 2002;77:152–5.PubMedGoogle Scholar
- World Health Organization. Meningococcal meningitis. Wkly Epidemiol Rec. 2003;78:294–6.PubMedGoogle Scholar
- Brown V, Moren A, Paquet C. Rapid health assessment of refugee or displaced populations. 2nd ed. Paris: Médecins sans Frontières; 1999. p. 28.
- Riou JY, Guibourdenche M. Laboratory methods: Neisseria and Branhamella. Paris: Editions Pasteur; 1993.
- Wilson IG. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–51.PubMedGoogle Scholar
- Sanou I, Ouedrago-Traore R, Ki-Zerbo GA, Bicaba I, Kam L, Sangare L, W135 meningococcal meningitis: study of 148 cases observed in 2002 and 2003 at the National Teaching Hospital of Ouagadougou, Burkina Faso. Med Trop (Mars). 2006;66:137–42.PubMedGoogle Scholar
- Djibo S, Njanpop Lafourcade BM, Boisier P, Moussa A, Kobo G, Sidikou F, Evaluation of the Pastorex meningitis kit for the rapid identification of Neisseria meningitidis serogroups A and W135. Trans R Soc Trop Med Hyg. 2006;100:573–8. DOIPubMedGoogle Scholar
- Borel T, Rose AM, Guillerm M, Sidikou F, Gerstl S, Djibo A, High sensitivity and specificity of the Pastorex latex agglutination test for Neisseria meningitidis serogroup A during a clinical trial in Niger. Trans R Soc Trop Med Hyg. 2006;100:964–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 6—June 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Philippe J. Guerin, Scientific Director, Epicentre, 8 rue St Sabin, Paris 75011, France;
Top