Volume 14, Number 11—November 2008
CME ACTIVITY - Research
Antimicrobial Drug Use and Resistance in Europe
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.com/cme/eid; (4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants will be able to:
- Identify the classes of antimicrobial drugs most commonly used in Europe.
- Describe patterns of antimicrobial drug use across regions in Europe.
- Identify the most widely used antimicrobial drugs by country in Europe.
- List European countries that show the highest antimicrobial drug resistance proportions.
- Describe the association between antimicrobial drug use and the emergence of resistance.
CME Editor
Anne Mather, Technical Writer-Editor, Emerging Infectious Diseases. Disclosure: Anne Mather has disclosed no relevant financial relationships.
CME Author
Désirée Lie, MD, MSEd, Clinical Professor, Family Medicine, University of California, Orange, California, USA; Director, Division of Faculty Development, UCI Medical Center, Orange. Disclosure: Désirée Lie has disclosed no relevant financial relationships.
Authors
Disclosures: Nienke van de Sande-Bruinsma, MSc, PhD; Hajo Grundmann, MD, PhD; Didier Verloo, DVM; Edine Tiemersma, PhD; Jos Monen, MSc; Herman Goossens, MD, PhD; and Matus Ferech, MSc, PhD, have disclosed no relevant financial relationships.
Abstract
Our study confronts the use of antimicrobial agents in ambulatory care with the resistance trends of 2 major pathogens, Streptococcus pneumoniae and Escherichia coli, in 21 European countries in 2000–2005 and explores whether the notion that antimicrobial drug use determines resistance can be supported by surveillance data at national aggregation levels. The data obtained from the European Surveillance of Antimicrobial Consumption and the European Antimicrobial Resistance Surveillance System suggest that variation of consumption coincides with the occurrence of resistance at the country level. Linear regression analysis showed that the association between antimicrobial drug use and resistance was specific and robust for 2 of 3 compound pathogen combinations, stable over time, but not sensitive enough to explain all of the observed variations. Ecologic studies based on routine surveillance data indicate a relation between use and resistance and support interventions designed to reduce antimicrobial drug consumption at a national level in Europe.
For the past 60 years, antimicrobial chemotherapy has been the mainstay of medical intervention against infectious diseases caused by bacterial pathogens. The continuous decline of therapeutic effectiveness as a result of extensive use of antimicrobial chemotherapy has been long predicted and seems inescapable (1). Many surveillance efforts have over the last decade (1997–2007) drawn attention to this phenomenon (2–5). At the same time, the once-abundant supply of new and improved antimicrobial compounds has worn thin, as drug development becomes increasingly challenging and pharmaceutical companies invest in more lucrative markets (6). It is therefore critical to realize that antimicrobial drug effectiveness, widely accepted as a common good, cannot be taken for granted and that such substances are increasingly attaining the status of nonrenewable resources.
Our study confronts the population-adjusted use of antimicrobial agents in ambulatory care with the resistance trends of 3 compound pathogen combinations in 21 European countries over a period of 6 years (2000–2005). This initial study was made possible by combining data from the 2 most comprehensive European surveillance systems on antimicrobial drug consumption and resistance, the European Surveillance of Antimicrobial Consumption (ESAC) (7) and the European Antimicrobial Resistance Surveillance System (EARSS) (8). We present an authoritative joint analysis of these 2 comprehensive databases. At this highly aggregated level, data are not sensitive enough to unravel the complex interaction between prescribing and resistance. The goal of this study is to give an overview of the situation in the European region and explore whether a relationship between antimicrobial drug use and resistance can be supported by empirical data pooled at national levels.
Consumption of Antimicrobial Agents
ESAC collects data on antimicrobial drug use in ambulatory care and hospital care in Europe. Currently, 24 countries report data on ambulatory care consumption to ESAC (9). Prescribed drugs are grouped by the active substance as the number of defined daily doses (DDD) per 1,000 inhabitants (DID) according to the World Health Organization definition of Anatomical Therapeutic Chemical Classification (ATC) defined daily dose (ATC-DDD version 2005 (10). A complete description of the data providers and details of the methods used by ESAC have been published (7,11,12). The performance and methodologic approach of the ESAC system, which aimed to collect comparable and reliable data on antimicrobial drug use, were studied by Vander Stichele et al. (7). The collected data were screened for bias caused by errors in assigning medicinal product packages to the ATC; errors in calculations of DDD per package; bias by over-the-counter sales and parallel trade; and bias in ambulatory care/hospital care mix. The study indicated that of the 31 participating countries, 21 delivered ambulatory care data suitable for cross-national comparison (7).
For the present study, the total country-specific antimicrobial drug use in ambulatory care and a breakdown into the following major antimicrobial classes were extracted from the ESAC database: penicillins (J01C); other β-lactam antimicrobial agents (cephalosporins, monobactams and carbapenems, J01D); macrolides, lincosamines, and streptogramins (MLS-class, J01F); and fluoroquinolones (J01MA).
Resistance to Antimicrobial Agents
EARSS performs continuous surveillance of antimicrobial drug susceptibility for 7 major bacterial pathogens that cause invasive infections. Data are provided by >900 microbiologic laboratories that serve ≈1,400 hospitals from 32 countries with an overall hospital catchment population estimated to include >100 million inhabitants (13). All EARSS participating laboratories perform routine antimicrobial drug susceptibility tests according to standard protocols (14) and interpret their susceptibility results according to harmonized national and international guidelines as sensitive, intermediately resistant, and resistant (15). More details about the data acquisition and analysis have been published elsewhere (13,16,17). The antimicrobial susceptibility test (AST) results reported by the laboratories are collected by using standardized protocols as described in the EARSS manual (www.rivm.nl/earss). Data that do not meet the requirements of these species-specific protocols are not accepted. To assess the comparability of results between laboratories participating in EARSS, an external quality assessment exercise is organized every year. A set of 6 strains is provided to each laboratory in collaboration with the UK National External Quality Assurance Scheme. These exercises illustrate that routinely reported results, as collected by EARSS, have sufficient accuracy to provide good estimates of overall resistance prevalences and trends (18).
For the present study, AST results of primary blood culture isolates of Escherichia coli and Streptococcus pneumoniae were extracted from the EARSS database to determine the proportions of penicillin- and erythromycin-nonsusceptible S. pneumoniae (PNSP and ENSP, respectively) and proportions of fluoroquinolone-resistant Escherichia coli (FQRE) bacteria. Nonsusceptible isolates included both intermediate resistant and resistant isolates. A country-specific resistance score was calculated as the sum of the quartile ranks of resistance against all 3 compound pathogen combinations (PNSP, ENSP, and FQRE). For trend analysis of resistance proportions per country over time, the Cochrane-Armitage trend test was used.
Ecologic Analysis
The strength of association between antimicrobial drug use and resistance was determined by univariate and multiple linear regression analysis. The proportion of resistance (R) in a country was transformed to the natural logarithm of the odds of resistance (ln[R/1–R]), to get a range from –∞ to +∞. The log odds of resistance (as the dependent variable) can then be expressed as a simple linear function of the independent variable (consumption) (19,20). To give equal weight to small countries with flawless data collection and not give the unequal weight to larger countries with sometimes less-optimal data, the linear regression analysis was not weighed.
To determine the delay between antimicrobial use and resistance, proportions of PNSP, ENSP, and FQRE for 2002–2005 were correlated with the consumption of different antimicrobial drug classes in the same year and the 2 years before. This resulted in 11 different exposure-outcome intervals for each compound–pathogen combination. For further multivariate analysis, the interval with the median correlation coefficient was regarded as representative for the association found in the overall study period.
Only the countries that reported volumes of antimicrobial drug prescriptions in ambulatory care from 2000 through 2004 and susceptibility data for the selected compound–pathogen combinations from 2002 through 2005 were included for linear regression analysis. Countries that provided yearly susceptibility data for <20 isolates were excluded. Data analysis was conducted by using SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA).
Consumption of Antimicrobial Agents
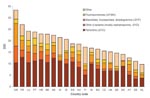
We included in the study 21 European countries, which provided data on the use of antimicrobial agents in ambulatory care to the ESAC database for the period 2000–2004 (including the 15 long-standing European Union (EU) member states). These also included 3 of the 10 nations that joined the EU in May 2004, the Czech Republic, Slovakia, and Slovenia; 2 applicant countries, Bulgaria and Croatia; and 1 European Free Trade Association country, Iceland. Total outpatient antimicrobial drug use differed significantly between countries. Use tends to be low in northern, moderate in central, and high in southern Europe and varied by a factor of 3.4 between Greece (33.4 DID) and the Netherlands (9.7 DID) in 2004 (Figure 1, Table 1).
During the observation period (2000–2004), antimicrobial drug use decreased (>15%) in Bulgaria, Czech Republic, France, and Germany and increased (>15%) in Croatia, Denmark, Greece, and Ireland. Penicillins (including broad-spectrum penicillins, ATC category J01C) represented the most widely used antimicrobial class in Europe. This class showed consumption patterns similar to the total outpatient antimicrobial drug use, as did the second most widely used category, which consists mainly of macrolides but also includes lincosamines and streptogramins (MLS class, ATC category J01F). The third most widely used ATC category (J01D, other β-lactams) consists of cephalosporins, monobactams, and carbapenems. Cephalosporins make up the bulk of the antimicrobial agents included in this group. Antimicrobial agents belonging to this category are more commonly used in hospitals; however, in some countries they are also extensively prescribed in ambulatory care. For this reason, use rates in Europe varied >100-fold between countries. The use of this ATC category decreased by >15% in 8 countries but increased in Slovenia. Fluoroquinolones hold the fourth position in the European market but showed the most dynamic increase, with growth rates of >15% in almost half of all countries (10/21). In terms of overall control of antimicrobial drug consumption, France most consistently reduced its use of 3 of the 4 most frequently prescribed antimicrobial drug classes (Figure 1, Table 1).
Resistance to Antimicrobial Agents
Large differences in the proportions of resistance were reported for the same countries. The highest antimicrobial drug resistance was found in Spain, Hungary, and France and the lowest in Sweden and the Netherlands in 2005 (Figure 2). Resistance proportions in 2005 differed by a factor of 27.7 for PNSP between France (36%) and the Netherlands (1.3%), by 20.5 for ENSP between France (41%) and the Czech Republic (2%), and by 9.7 for fluoroquinolone resistance in E. coli between Portugal (29%) and Iceland (3%). From 2001 through 2005, resistance levels remained relatively stable for PNSP but increased for the other 2 compound pathogen combinations (Table 2). Spain and the United Kingdom were the only countries that reported any significant decrease in antimicrobial drug resistance rates. In Spain, penicillin nonsusceptibility fell from 37% to 25% and in the United Kingdom, from 5% to 3.8%. For ENSP a significant increase was observed in Hungary (from 19% to 37%), Finland (from 12% to 20%), and the Netherlands (5% to 11%). The most consistent trend was observed for fluoroquinolone resistance in E. coli, which increased in most European countries (Table 2).
Combining Antimicrobial Drug Use with Susceptibility Data
Greece (33.0 DID), France (27.1 DID), Luxembourg (24.2 DID), Portugal (23.8 DID), Croatia (23.0 DID), and Belgium (22.9 DID) were the countries that reported the highest use of antimicrobial agents in ambulatory care. Four of these high-consumer countries—France, Luxemburg, Belgium, and Portugal—were also among the 6 countries with the highest resistance proportions. Croatia occupied an intermediate resistance rank, owing to more modest levels in fluoroquinolone resistance. For Greece, susceptibility data for S. pneumoniae were not available, which precluded a meaningful ranking. Although Spain (18.7 DID) and Hungary (18.6 DID) were not among the countries with the highest use of antimicrobial agents, both countries did have the highest antimicrobial drug resistance proportions in 2005. The United Kingdom (15.2 DID), Sweden (15 DID), Denmark (14.1 DID), Austria (12.5 DID), Germany (11 DID), and the Netherlands (10 DID) reported the lowest antimicrobial drug use in outpatient settings. Of these, Sweden, the Netherlands, Denmark, and the United Kingdom also were among the 6 countries with the lowest resistance proportions. Germany and Austria reported medium to high rates especially for ENSP (17% and 15%, respectively) and FQRE (23% and 19%, respectively) (Figures 1, 2). Because inspection of the data suggested a relation between antimicrobial drug consumption and resistance, this assumption was formally tested by using simple linear regression.
Because little is known about the delay that can be expected between the change in antimicrobial drug exposure and its effect on antimicrobial resistance at a population level, different intervals were chosen to explore the potential association between use and resistance. Intervals were explored for same-year data, a 1-year delay, and a 2-year delay between exposure and outcome. Thus, the consumption data available for 2000 through 2004 and resistance data for 2002–2005 provided the means to explore the correlation coefficients of 11 exposure-outcome intervals. Only the 17 countries that provided data for all years were included in the linear regression analysis. Table 3 shows the range and median correlation coefficient for all exposure-outcome intervals. Since no statistically significant time dependence was observed, the median correlation coefficient was regarded as representative for the association found for the entire study period (Table 3).
The occurrence of PNSP in European countries correlated with the country-specific use of penicillins, which explained 61% of the observed variance (p<0.01) (Figure 3). The second best correlation was provided by the total antimicrobial drug use in ambulatory care, which explained 46% of the observed variance (p<0.01). Both associations were robust and remained significant, regardless of the interval between the ascertainment of antimicrobial drug use and the recording of antimicrobial resistance. A notably less consistent association was found when we correlated the use of MLS-class antimicrobial agents or fluoroquinolones with the occurrence of PNSP (Table 3). ENSP occurrence in Europe correlated most compellingly with the country-specific use rate of ATC category J01D (other β-lactams), which explained 48% of the observed variance (p<0.01) (Table 3). However, this effect appeared to be confounded by the use of MLS-class antimicrobial agents and fluoroquinolones. By fitting use data for these antimicrobial agents into the model, the effect estimates for the former decreased by 40% (Table 4), indicating that part of the effect attributed to the use of other β-lactam antimicrobial agents appeared to be exerted by MLS-class antimicrobial agents and fluoroquinolones.
Proportions of FQRE in European countries were best explained by the country-specific use data for fluoroquinolones. Fluoroquinolone consumption as reported to the ESAC network explained 36% of the variance observed in EARSS data (p<0.01; Figure 4). This effect appeared to be specific and was not associated or confounded by consumption of the other antimicrobial classes.
We compared the trends in antimicrobial drug consumption patterns and the antimicrobial drug resistance proportions for 2 major pathogens, S. pneumoniae and E. coli, in Europe from 2000 through 2005. Antimicrobial drug use in outpatient settings was ascertained by the most comprehensive network for European surveillance of antimicrobial consumption (ESAC), and antimicrobial resistance data were obtained from the European surveillance system EARSS). The data suggested that in Europe the variation of consumption coincides with the occurrence of resistance at country level. Using simple linear regression analysis, we formally explored whether a relation between country-specific antimicrobial drug use and antimicrobial resistance can be inferred at national aggregation levels and found that the association between antimicrobial drug use and resistance was specific and robust for 2 of the 3 compound pathogen combinations under study, stable over time, but not sensitive enough to explain all of the observed variation.
There was a high degree of consistency between penicillin use and penicillin nonsusceptibility in pneumococci as well as for fluoroquinolone use and an increase in fluoroquinolone resistance in E. coli. Simple linear regression showed that these effects were highly specific and robust, as inclusion of the use of other antimicrobial substances did not improve correlation or was not confounding the overall effect estimates (Tables 3, 4; Figures 3, 4). The mechanisms for acquiring resistance against both substances have some features in common. These include successive alterations of chromosomally located genes by either homologous recombination or point mutations, resulting in a stepwise modification of the molecular targets, which first leads to reduced susceptibility and eventually to complete resistance (21,22). In contrast to many other resistance mechanisms, no mobile genetic elements are involved, and a physical linkage to other resistance determinants is unlikely. It is therefore expected that before phenotypes with stable combined resistance evolve, antimicrobial drug selection will specifically favor homologous resistance.
A nonhomologous effect was observed in the case of ENSP, since the variance in ENSP occurrence was best explained by the country-specific use rates of the ATC category of other β-lactams, consisting mainly of cephalosporins. This observation could be either causal, coincidental, or both. In fact, the results of multiple regression models indicate a degree of confounding, as part of the effect attributed to other β-lactams could be explained by MLS-class antimicrobial agents and fluoroquinolones (Table 4). This confounding effect implies that the effect of other β-lactams is mixed with the effect of MLS-class antimicrobial agents and fluoroquinolones used. Data recorded by ESAC suggest that most countries with high use of other β-lactams also have a high consumption of MLS-class antimicrobial agents (r = 0.78, p<0.01) as well as fluoroquinolones (r = 0.65, p<0.01). Moreover, countries with the highest levels of other β-lactam use—such as Luxembourg, Croatia, Portugal, Belgium, and France—and high levels of ENSP (23%, 19%, 20%, 31%, and 41%) also reported high levels of combined nonsusceptibility to both erythromycin and penicillin (12%, 9%, 10%, 9%, and 32%). Any increase in selection pressure exerted by β-lactams would also co-select for ENSP under these conditions of combined nonsusceptibility, which could also explain the absence of a direct relationship between use of MLS-class antimicrobial agents and ENSP.
For all compound pathogen combinations that showed significant correlations, the association between the volume of antimicrobial agents used and proportions of resistance was for the most part stable, i.e., independent of the time lag between recording of consumption and the recording of resistance (Table 3). This is not surprising because in the absence of nationwide interventions that would abruptly change the use pattern for an entire country, no major trend changes would be expected, or as other authors have already stated, it is likely that a country with more use or resistance than others in one year, will also have more use or resistance in the next (19). Likewise, the steady decline in the consumption in some of the antimicrobial drug classes such as penicillins, as happened in the Czech Republic, France, Germany, and Slovakia, was not reflected by a concomitant decline of penicillin resistance in the pathogens under selective pressure. Mathematical models as well as empirical data suggest that after a reduction in prescribing, resistance will take longer to decline than it took to rise (23). In the same way, no decline in resistance against co-trimoxazole was observed in the United Kingdom even 10 years after it abandoned its prescribing, which in this instance was attributed to the co-selection of genetically linked resistance determinants by alternative antimicrobial pressure (24). EARSS data show a significant reduction of penicillin resistance in Spain (25) and the United Kingdom over the past 5 years (2001–2005), however, no corresponding decline in penicillin use has become apparent that could explain this favorable development (Tables 1, 2). Alternatively, data aggregated at country level by established surveillance networks may not be sensitive enough to identify subtle changes in the complex interaction between antimicrobial drug prescribing and resistance.
EARSS data consist of antimicrobial drug resistance proportions of bacteria that cause invasive bloodstream infections but do not include information from other potentially relevant patient materials. This omission limits the wealth of data but improves the comparability between participating laboratories because it reduces bias introduced by differential case ascertainment. S. pneumoniae is the main cause of community-acquired bacteremic pneumonia (26), and invasive E. coli infections are mainly caused by the translocation of intestinal colonizing strains (27). Thus, we believe that resistance among S. pneumoniae and E. coli blood culture isolates would sufficiently reflect the ecological pressure exerted by the antimicrobial drug use in outpatient settings.
There is little doubt that antimicrobial drug consumption is important in the dissemination of antimicrobial drug resistance. However, additional or alternative factors need to be taken into account (28).
We could not control for country-specific differences in hygiene, diagnostic habits, community infection control, and vaccination policies that could provide alternative explanations for some of the observed differences. Moreover, inconsistencies in the sampling population covered by the 2 surveillance systems may introduce inaccuracies that hamper the internal validity of this type of analysis (29). In general, data at this high aggregation level are probably not sensitive enough to reflect subtle changes in the complex interaction between antimicrobial drug prescribing and resistance. In this respect, increasing the geographic resolution of data collection by addressing antimicrobial drug use and resistance at the level of health districts would improve the analysis and degree of causal inference that these studies could provide. A higher geographic resolution could also foster interventions by making local extremes of use apparent. However, despite these drawbacks, the data suggest that a specific, robust, and stable association exists between antimicrobial drug use and the occurrence of resistance at country level in the European Union. Our results therefore support interventions that encourage healthcare professionals and healthcare authorities to take firm steps toward promoting prudent use and careful restriction of antimicrobial drug prescription and to monitor the effect of these interventions toward the restoration of the antiinfective activity essential to the success of modern medicine.
Dr van de Sande-Bruinsma has been part of the EARSS management team since 2001. Her research interest is antimicrobial drug resistance.
Acknowledgment
EARSS and ESAC are funded by the European Commission. EARSS is cofinanced by the Dutch Ministry of Health, Welfare and Sports, the Netherlands.
References
- Rammelkamp M. Resistance of Staphylococcus aureus to the action of penicillin. Proc Soc Exp Biol Med. 1942;51:386–9.
- Felmingham D, White AR, Jacobs MR, Appelbaum PC, Poupard J, Miller LA, The Alexander Project: the benefits from a decade of surveillance. J Antimicrob Chemother. 2005;56(Suppl 2):ii3–21. DOIPubMedGoogle Scholar
- Jones RN. Global epidemiology of antimicrobial resistance among community-acquired and nosocomial pathogens: a five-year summary from the SENTRY Antimicrobial Surveillance Program (1997-2001). Semin Respir Crit Care Med. 2003;24:121–34. DOIPubMedGoogle Scholar
- Mackenzie FM, Bruce J, Van Looveren M, Cornaglia G, Gould IM, Goossens H. Antimicrobial susceptibility testing in European hospitals: report from the ARPAC study. Clin Microbiol Infect. 2006;12:1185–92. DOIPubMedGoogle Scholar
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis. 2007;57:7–13. DOIPubMedGoogle Scholar
- Finch R, Hunter PA. Antibiotic resistance—action to promote new technologies: report of an EU intergovernmental conference held in Birmingham, UK, 12–13 December 2005. J Antimicrob Chemother. 2006;58(Suppl 1):i3–22. DOIPubMedGoogle Scholar
- Vander Stichele RH, Elseviers MM, Ferech M, Blot S, Goossens H. European surveillance of antimicrobial consumption (ESAC): data collection performance and methodological approach. Br J Clin Pharmacol. 2004;58:419–28. DOIPubMedGoogle Scholar
- Bronzwaer SL, Goettsch W, Olsson-Liljequist B, Wale MC, Vatopoulos AC, Sprenger MJ. European Antimicrobial Resistance Surveillance System (EARSS): objectives and organisation. Euro Surveill. 1999;4:41–4.PubMedGoogle Scholar
- Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother. 2006;58:401–7. DOIPubMedGoogle Scholar
- World Health Organization. The anatomical therapeutic chemical classification system with defined daily doses (AT/DDD). Oslo: The Organization; 2005.
- Vander Stichele RH, Elseviers MM, Ferech M, Blot S, Goossens H. Hospital consumption of antibiotics in 15 European countries: results of the ESAC retrospective data collection (1997-2002). J Antimicrob Chemother. 2006;58:159–67. DOIPubMedGoogle Scholar
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87.PubMedGoogle Scholar
- EARSS management team. EARSS annual report 2005. Bilthoven (the Netherlands): National Institute for Public Health and the Environment; 2006.
- EARSS Management Team. EARSS manual 2005 [cited 2006 Nov 30]. Available from http://www.rivm.nl/earss/Images/Earss%20manual2005_tcm61-21261.pdf
- Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Odenholt I, European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006;12:501–3. DOIPubMedGoogle Scholar
- Tiemersma EW, Bronzwaer SL, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis. 2004;10:1627–34.PubMedGoogle Scholar
- Bruinsma N, Kristinsson KG, Bronzwaer S, Schrijnemakers P, Degener J, Tiemersma E, Trends of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 2004;54:1045–50. DOIPubMedGoogle Scholar
- Bronzwaer S, Buchholz U, Courvalin P, Snell J, Cornaglia G, de Neeling A, Comparability of antimicrobial susceptibility test results from 22 European countries and Israel: an external quality assurance exercise of the European Antimicrobial Resistance Surveillance System (EARSS) in collaboration with the United Kingdom National External Quality Assurance Scheme (UK NEQAS). J Antimicrob Chemother. 2002;50:953–64. DOIPubMedGoogle Scholar
- Lipsitch M. The rise and fall of antimicrobial resistance. Trends Microbiol. 2001;9:438–44. DOIPubMedGoogle Scholar
- Bronzwaer SL, Cars O, Buchholz U, Mölstad S, Goettsch W, Veldhuijzen IK, A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8:278–82.PubMedGoogle Scholar
- Charpentier E, Tuomanen E. Mechanisms of antibiotic resistance and tolerance in Streptococcus pneumoniae. Microbes Infect. 2000;2:1855–64. DOIPubMedGoogle Scholar
- Chenia HY, Pillay B, Pillay D. Analysis of the mechanisms of fluoroquinolone resistance in urinary tract pathogens. J Antimicrob Chemother. 2006;58:1274–8. DOIPubMedGoogle Scholar
- Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96:1152–6. DOIPubMedGoogle Scholar
- Enne VI, Livermore DM, Stephens P, Hall LM. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet. 2001;357:1325–8. DOIPubMedGoogle Scholar
- Garcia-Rey C, Bouza E, Aguilar L, Garcia-de-Lomas J, Baquero F. Evolution of penicillin and erythromycin co-resistance in Streptococcus pneumoniae in Spain. Int J Antimicrob Agents. 2003;22:541–4. DOIPubMedGoogle Scholar
- Moellering RC Jr, Craig W, Edmond M, Farrell DJ, Ferraro MJ, File TM Jr, Clinical and public health implications of macrolide-resistant Streptococcus pneumoniae. J Chemother. 2002;14(Suppl 3):42–56. DOIPubMedGoogle Scholar
- Perrin M, Donnio PY, Heurtin-Lecorre C, Travert MF, Avril JL. Comparative antimicrobial resistance and genomic diversity of Escherichia coli isolated from urinary tract infections in the community and in hospitals. J Hosp Infect. 1999;41:273–9. DOIPubMedGoogle Scholar
- Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg Infect Dis. 2002;8:1460–7.PubMedGoogle Scholar
- Steinke D, Davey P. Association between antibiotic resistance and community prescribing: a critical review of bias and confounding in published studies. Clin Infect Dis. 2001;33(Suppl 3):S193–205. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.com/cme/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Microbial Interactions during Upper Respiratory Tract Infections
CME Questions
1. Which of the following classes of antimicrobial drugs is least likely to be used in European countries?
A. Lincosamides
B. Fluoroquinolones
C. Cotrimoxazole
D. Macrolides
2. Which of the following regions in Europe has the highest outpatient utilization of antimicrobial drugs?
A. Southern
B. Northern
C. Eastern
D. Central
3. Which of the following is the most widely used antimicrobial drug class in Europe?
A. Macrolides
B. Penicillins
C. Nonpenicillin beta-lactams
D. Fluoroquinolones
4. Which of the following European countries showed both the greatest use of antimicrobial drugs in ambulatory care and the highest resistance proportions?
A. France
B. Croatia
C. Italy
D. Greece
5. Among European countries with high antimicrobial drug resistance rates, a robust and consistent association was most likely to be found between utilization and resistance for which of the following drugs?
A. Penicillins and macrolides
B. Lincosamides and macrolides
C. Penicillins and streptogramins
D. Penicillins and fluoroquinolones
Activity Evaluation
| 1. The activity supported the learning objectives. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
| 1 |
2
|
3
|
4
|
5
|
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
| 1 |
2
|
3
|
4
|
5
|
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
| 1 |
2
|
3
|
4
|
5
|
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
| 1 |
2
|
3
|
4
|
5
|
1National representatives of these 2 project groups in 2005 were as follows: Austria: H. Mittermayer, S. Metz, W. Koller (European Antimicrobial Resistance Surveillance System [EARSS]); Belgium: E. Hendrickx (EARSS), H. Goossens; Bulgaria: B. Markova; Croatia: A. Tambic-Andrasevic, Igor Francetic (European Surveillance of Antimicrobial Consumption [ESAC]), S. Kalenic (EARSS); Cyprus: D. Bagatzouni (EARSS); Czech Republic: P. Dvorak (ESAC), P. Urbaskova (EARSS); Denmark: D. Monnet, A. Anker Nielsen (ESAC); Estonia: P. Naaber (EARSS); Finland: P. Huovinen (ESAC), P. Paakkari (ESAC), O. Lyytikainen (EARSS), A. Nissinen (EARSS); France: P. Maugendre (ESAC), D. Guillemot (ESAC), B. Coignard, (EARSS), V. Jarlier (EARSS); Germany: W. Kern (ESAC), H. Schroeder (ESAC), W. Witte (EARSS), K. Heckenbach (EARSS); Greece: H. Giamarellou (ESAC), A. Antoniadou (ESAC), A. Tsakris (EARSS), A. Vatopoulos (EARSS); Hungary: G. Ternak (ESAC), M. Fuzi (EARSS); Iceland: K. Kristinsson; Ireland: E. Smyth (ESAC), R. Cunney (ESAC), D. Igoe (EARSS), O. Murphy (EARSS); Israel: R. Raz (EARSS); Italy: G. Cornaglia, A. Pantosti (EARSS), P. D’Ancona (EARSS); Latvia: S. Berzina (ESAC), A. Balode (EARSS); Lithuania: R. Valenteliene (ESAC), J. Miciulevicience; Luxembourg: R. Hemmer, M. Bruch (ESAC); Malta: M. Borg, P. Zarb (ESAC); the Netherlands: R. Janknegt (ESAC), M. Filius (ESAC), H. de Neeling (EARSS), E. Tiemermsa, J Degener (EARSS); Norway: H. Salvesen Blix (ESAC), A. Hoiby (EARSS), G. Simonsen (EARSS); Poland: W. Hryniewicz, P. Grzesiowski; Portugal: L. Caldeira (ESAC), M. Canica (EARSS); Romania: I. Codita; Slovakia: V. Foltan (ESAC), T. Tesar (ESAC), L. Langsadl (EARSS); Slovenia: M. Cizman (ESAC), M. Mueller-Premru (EARSS), J. Kolman (EARSS); Spain: J. Campos, F. Baquero (EARSS); Sweden: O. Cars (ESAC), G. Skoog (ESAC), B. Liljequist (EARSS), G. Kahlmeter (EARSS); Turkey: S. Unal (ESAC), D. Gür (EARSS); United Kingdom: P. Davey (ESAC), A. Johnson (EARSS), R. Hill (EARSS), H. Hughes (EARSS), M. Coyne (EARSS).
Related Links
Table of Contents – Volume 14, Number 11—November 2008
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Hajo Grundmann, National Institute of Public Health and the Environment, Centre for Infectious Diseases Epidemiology, A. van Leeuwenhoeklaan 9, 3720 BA Bilthoven, the Netherlands
Top