Volume 14, Number 12—December 2008
Dispatch
Transmission of Atypical Bovine Prions to Mice Transgenic for Human Prion Protein
Figure 1
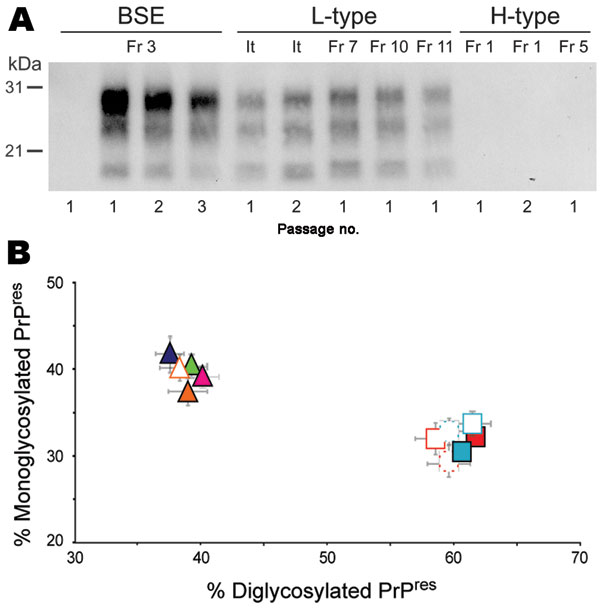
Figure 1. Protease-resistant prion protein (PrPres) in the brains of human PrP transgenic mice infected with atypical or zoonotic bovine spongiform encephalopathy (BSE) agents. A) Representative Western blot analysis of PrPres extracted (for detailed protocol, see 7) from brain homogenates of mice at terminal stage of disease or at end of lifespan after serial transmission of atypical (L-type and H-type) or classical BSE isolates. The amount of equivalent brain tissue loaded onto the gels was 0.01 mg (BSE; Fr3 isolate, 2nd and 3rd passage), 0.3 mg (L-type and 1st passage of BSE), and 10 mg (PrPres-negative samples). Anti-PrP monoclonal antibody Sha31 was used for PrPres detection. Immunoreactivity was determined by chemiluminescence. B) Ratio of diglycosylated and monoglycosylated PrPres species in the brains of mice after serial transmission of L-type or BSE isolates (data plotted as means ± standard error of the mean). Primary passage of L-type isolates are represented as triangles (orange, It; blue, Fr7; green, Fr10; pink, Fr11) and BSE as squares (light blue, Fr3; red, Ge). Passages are indicated by unfilled symbols of the same color (solid line, second passage; broken line, third passage). The ratio was determined after acquisition of PrPres chemiluminescent signals with a digital imager as previously described (7). Note the distinct glycoform ratio between L-type and BSE groups. It, Italy; Fr, France; Ge, Germany.
References
- Wadsworth JD, Collinge J. Update on human prion disease. Biochim Biophys Acta. 2007;1772:598–609.
- Grassi J, Creminon C, Frobert Y, Fretier P, Turbica I, Rezaei H, Specific determination of the proteinase K-resistant form of the prion protein using two-site immunometric assays. Application to the post-mortem diagnosis of BSE. Arch Virol Suppl. 2000;16:197–205.PubMedGoogle Scholar
- Biacabe AG, Laplanche JL, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 2004;5:110–5. DOIPubMedGoogle Scholar
- Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 2004;101:3065–70. DOIPubMedGoogle Scholar
- Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, Hoffmann C, Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet Microbiol. 2006;117:103–16. DOIPubMedGoogle Scholar
- Biacabe AG, Morignat E, Vulin J, Calavas D, Baron TG. Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerg Infect Dis. 2008;14:298–300. DOIPubMedGoogle Scholar
- Beringue V, Andreoletti O, Le Dur A, Essalmani R, Vilotte JL, Lacroux C, A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci. 2007;27:6965–71. DOIPubMedGoogle Scholar
- Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, Conversion of the BASE prion strain into the BSE strain: the origin of BSE? PLoS Pathog. 2007;3:e31. DOIPubMedGoogle Scholar
- Béringue V, Bencsik A, Le Dur A, Reine F, Lai TL, Chenais N, Isolation from cattle of a prion strain distinct from that causing bovine spongiform encephalopathy. PLoS Pathog. 2006;2:e112. DOIPubMedGoogle Scholar
- Baron T, Bencsik A, Biacabe AG, Morignat E, Bessen RA. Phenotypic similarity of transmissible mink encephalopathy in cattle and L-type bovine spongiform encephalopathy in a mouse model. Emerg Infect Dis. 2007;13:1887–94.PubMedGoogle Scholar
- Baron TG, Biacabe AG, Bencsik A, Langeveld JP. Transmission of new bovine prion to mice. Emerg Infect Dis. 2006;12:1125–8.PubMedGoogle Scholar
- Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol. 2008;82:3697–701. DOIPubMedGoogle Scholar
- Béringue V, Le Dur A, Tixador P, Reine F, Lepourry L, Perret-Liaudet A, Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLoS One. 2008;3:e1419. DOIPubMedGoogle Scholar
- Hecker R, Taraboulos A, Scott M, Pan KM, Yang SL, Torchia M, Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 1992;6:1213–28. DOIPubMedGoogle Scholar
- Béringue V, Vilotte JL, Laude H. Prion agent diversity and species barrier. Vet Res. 2008;39:47. DOIPubMedGoogle Scholar