Volume 14, Number 7—July 2008
Research
Toxinotype V Clostridium difficile in Humans and Food Animals
Figure 2
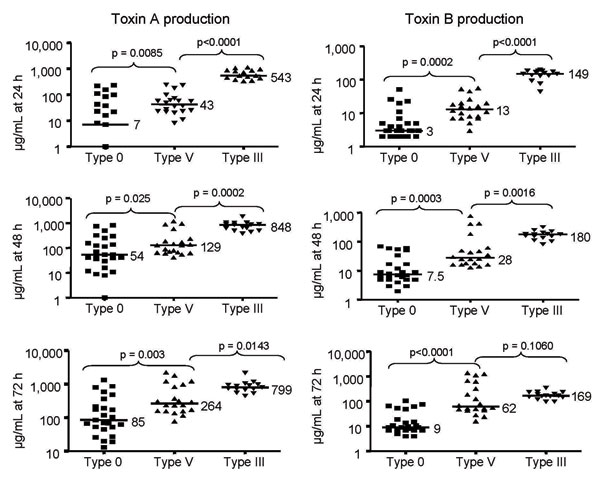
Figure 2. In vitro toxin production of toxinotype V Clostridium difficile isolates compared with epidemic toxinotype III and nonepidemic toxinotype 0 strains. Toxin A and Toxin B concentrations in micrograms per milliliter at 24, 48, and 72 h are shown for 25 toxinotype 0 isolates, 21 toxinotype V isolates (7 human; 14 animal), and 15 toxinotype III isolates. Horizontal lines indicate median values for each group and the p values are shown for comparison of the median toxin levels of toxinotype V isolates with toxinotype 0 and toxinotype III isolates.
Page created: July 12, 2010
Page updated: July 12, 2010
Page reviewed: July 12, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.