Volume 15, Number 2—February 2009
Research
Imported Malaria in Children in Industrialized Countries, 1992–2002
Abstract
Children account for an appreciable proportion of total imported malaria cases, yet few studies have quantified these cases, identified trends, or suggested evidence-based prevention strategies for this group of travelers. We therefore sought to identify numbers of cases and deaths, Plasmodium species, place of malaria acquisition, preventive measures used, and national origin of malaria in children. We analyzed retrospective data from Australia, Denmark, France, Germany, Italy, Japan, the Netherlands, Sweden, Switzerland, the United Kingdom, and the United States and data provided by the United Nations World Tourism Organization. During 1992–2002, >17,000 cases of imported malaria in children were reported in 11 countries where malaria is not endemic; most (>70%) had been acquired in Africa. Returning to country of origin to visit friends and relatives was a risk factor. Malaria prevention for children should be a responsibility of healthcare providers and should be subsidized for low-income travelers to high-risk areas.
Malaria is associated with high healthcare costs (1). Although several industrialized countries (e.g., most European countries, the United States, Australia, Japan) (2) are classified as malaria nonendemic, human migration and tourist travel to malaria-endemic regions are resulting in the importation of malaria (an estimated 30,000 cases/year) into these malaria-free countries (3). In the World Health Organization European Region, the number of imported cases rose from 1,500 in 1972 to 13,000 in 1999 (4). Children account for a considerable proportion of total malaria cases imported into the United States and Europe (5). International migration increased from 75 million in 1960 to 175 million in 2000 (6). Furthermore, the World Population Prospects report predicts a sharp increase in the number of persons who will migrate from southern (malaria-endemic) areas to northern (malaria-free) industrialized areas worldwide (7). This immigration trend also predetermines subsequent immigrant travel patterns. Immigrants returning to visit families in their home countries are at high risk for travel-related illness (8–12).
Malaria cases in children especially are increasing as more children travel and as the profile of immigrants changes (13–16). Our main study objectives were to evaluate the epidemiology of imported malaria in children in industrialized countries, identify trends and risk groups, and rank destinations according to malaria risk for children.
We collected retrospective data on imported malaria cases in children for the 11-year period January 1992 through December 2002. We requested data from 11 industrialized countries in which malaria is not endemic: Australia, Denmark, France, Germany, Italy, Japan, the Netherlands, Sweden, Switzerland, the United Kingdom, and the United States.
Malaria Cases
We defined imported malaria in a child as parasitologically confirmed malaria that had been acquired in a disease-endemic area by a person <18 years of age and that was diagnosed after clinical disease had developed and when the person was in an industrialized country where the disease was not endemic. Data were collated directly from the countries’ health authorities and consisted of aggregated case numbers of imported malaria in children by year (1992–2002), age group, sex, Plasmodium species, place of infection acquisition, number of deaths, national origin of patients, and preventive measures used during travel (i.e., chemoprophylaxis, bed nets, protective clothing, repellents).
We examined the distribution of malaria cases in children according to the variables of interest. Total cases were stratified according to Plasmodium species. On the basis of numbers of deaths and of Plasmodium falciparum cases, we calculated case-fatality ratio and 95% confidence intervals (CIs).
Traveler Statistics
The United Nations World Tourism Organization provided data on total numbers of travelers and extrapolated numbers of children <18 years of age who had traveled from >1 of the 11 industrialized countries in this study to malaria-endemic areas in Africa. Because exact numbers were not readily available, we assumed and used as proxy data the proportion of young travelers from the overall number of arrivals at specific African destinations. The assumption that 1 of 10 travelers is <18 years of age is consistent with the number of visits of young UK residents to African destinations in 2000. During that year, an estimated 138,000 persons in this age group accounted for 1,439,000 visits to Africa (17). The number of malaria cases in children per 10,000 visitors can be treated as a proxy. Using the number of child travelers as denominator, we calculated the rate of malaria cases acquired by children in the African regions and compared destination countries in Africa according to malaria risk for young travelers. Denominator data did not account for time spent in the malaria-transmission area. We also used surveillance data to attempt to determine nationality or country of origin (ethnicity) of the children with malaria.
Number of Cases
Of 17,009 reported malaria cases in children from the 11 industrialized countries studied, >75% were from only 3 countries (Table 1): France (n = 6,618), United Kingdom (n = 3,816; children <17 years of age), and United States (n = 2,614). The number of reported cases per year varied from 0 in Japan in 1996 to 1,096 in France in 1999. The number of cases registered in all contributing countries together was highest in 1999 (n = 2,233) and declined thereafter.
Among the different age groups, the largest overall percentage of cases occurred in those 15–17 years of age (18.1% of total cases) (Table 2). Analysis by age group showed heterogeneity between the countries. Japan and Australia showed high case rates; the 18-year age group accounted for almost 25% and 15% of all cases, respectively. Boys accounted for 55% of the total cases and predominated in all participating countries (data not shown).
Region of Malaria Acquisition
Of the 15,505 cases for which detailed data on country of acquisition were obtained, for all countries except Japan, >50% of cases were imported from Africa (Table 3); West Africa accounted for >50% of cases imported from Africa. Asia and Central and South America accounted for a small proportion of the imported malaria cases in children. Central and South America were responsible for a negligible number of infections, but in the United States, children accounted for 348 imported malaria cases (13% of all malaria cases in children [data not shown]). The predominant source of infections acquired in Asia was southern Asia (e.g., India, Pakistan, Sri Lanka) rather than Southeast Asia (e.g., Thailand, Indonesia, Vietnam, Malaysia, Philippines) (data not shown).
Case-Fatality Ratio
The case-fatality ratio for all countries was <0.4%; 3 countries (Italy, Sweden, and Japan) recorded no malaria-associated deaths in children during the period of observation (Table 4). Information about use of chemoprophylaxis in traveling children was limited. Among children with malaria, only 17.5% had taken chemoprophylaxis.
Plasmodium Species
Plasmodium species varied among the countries. The predominant species was P. falciparum, which accounted for 69.9% of all cases. The highest proportion of cases caused by P. falciparum (83.1%) was in France (Table 5).
Return to Native Country
High-risk malaria destinations reflect the migrant population in the source country. For France, >2 million travelers visited Africa; the overall rate of malaria acquisition for children was 22/10,000 arrivals in Africa and 110/10,000 arrivals in considerable-risk countries within Africa. However, travelers to specific countries had a huge comparative risk. Children from France who visited the Comoros islands (n = 10,460) had a malaria attack rate of 1,251/10,000. From France, the Comoros islands are a recognized destination for visiting friends and relatives; no other country evaluated reported any malaria cases for travelers to the Comoros. In comparison, large numbers of travelers from France, probably tourists, visit Kenya (n = 527,880), where the attack rate for the child travelers is 3.8/10,000 arrivals. The countries where children were most likely to acquire malaria were, in order of risk, Comoros (1,030 malaria cases/10,000 arrivals), Democratic Republic of Congo (778), Central African Republic (444), Guinea (308), Mali (203), Côte d’Ivoire (177), Congo (175), Nigeria (139), Bénin (134), Sierra Leone (127), Cameroon (109), Togo (102), and Ghana (100) and reflected the African nationality or origin of the immigrant communities in industrialized countries.
During 1992–2002, >17,000 cases of imported malaria in children were reported in 11 industrialized countries in which malaria is not endemic. Of all cases in children with known place of disease acquisition, >75% were acquired in Africa, mainly West Africa. P. falciparum was the dominant imported species; case-fatality ratio for all countries was <0.4%. Imported malaria in children is associated with travel, especially travel to visit friends and relatives, to high-risk malaria-endemic areas such as the Comoros islands and western and central African countries.
The strength of our study lies in the compilation of a large amount of data from national authorities, which enabled a global analysis. These data, coupled with data on arrivals in destination countries (18), enabled us to create a risk analysis for children traveling to malaria-endemic areas.
Limitations of our study include artifacts in malaria surveillance data and traveler statistics. Underreporting remains a problem in many countries (19), so our study could underestimate the true situation of imported malaria in children. Also, the quantity and quality of data received varied among countries and showed great heterogeneity despite efforts to standardize reporting in Europe (3).
Surveillance systems and malaria case definitions differ; some countries rely on laboratories or clinicians or both for source data (19). One country had data for children up to only 17 years of age, some countries had data for only part of the requested period, and some countries used extrapolated data.
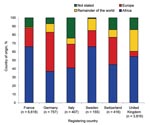
Nationality and ethnicity posed logistical problems for data analysis. In the absence of data on “reason for travel,” we assumed that ethnicity represented the group who traveled to visit friends and relatives in their native country, which might not necessarily be true for countries such as the United States. The Figure shows the origin of the malaria patients rather than nationality, for which data are unavailable or unreliable. Information concerning the use of chemoprophylaxis is collected infrequently, if at all.
In some countries, e.g., Germany, the Netherlands, and Australia, notification systems changed during the study period (20–22). Our collated cases have also been influenced by traveler’s choice of destination and use of preventive measures during travel, but other determinants included possible immunity or partial immunity of newly arrived immigrants to industrialized countries and number of travelers to malaria-endemic areas (23).
Statistics on traveler numbers, although imperfect, are the best available. To draw meaningful conclusions, we related numbers by destination country and source country to the corresponding visitation levels. Proxy data were used to estimate the percentage of young travelers to the various African destinations.
Data on arrivals need to be treated with caution because definitions, methods, and collection and compilation practices may differ from country to country. Many destination countries in Africa report arrivals by nationality and not by residency. With respect to malaria, method of reporting can imply that the number of visitors originating from the various source countries is higher than the actual number (nationals residing abroad might not be included) and that the number of malaria cases/10,000 children is overestimated. Furthermore, several destination countries have small focal areas where malaria transmission occurs, yet the denominator estimate included travel to the whole country, which may falsely lower the malaria risk estimate.
Our finding of a high rate of P. falciparum cases in France is consistent with findings of several studies about imported malaria in children in France (13,14,24,25). Castéla et al. describe malaria in France as essentially imported from Africa (13). Eloy et al. found that 90% of the 60 children with malaria at the Versailles Hospital between January 1997 and December 2001 were of African origin and that 84% had P. falciparum malaria (26).
In Italy, between 1989 and 1997, a steady increase in the number of cases among foreigners in all age groups has been reported, while cases among Italian nationals have remained stable (27). In 2000, foreign nationals represented almost 73% of total imported malaria cases in all age groups; of these, 93% were African (28). In our study, 41% of children with malaria registered in Italy were of African nationality. Place of acquisition of P. falciparum infection was Africa for >93% of children; >75% of cases were acquired in West Africa.
In the United Kingdom in the 1970s, a large proportion of imported malaria cases were attributable to P. vivax and associated with a large number of immigrants from India and Pakistan. Since the 1980s, however, the situation of imported malaria in the United Kingdom has changed (29,30); the overall ratio of cases caused by P. falciparum to those caused by P. vivax has increased from ≈37% in the mid-1980s to 55% in the mid-1990s (15). Of the 3,816 cases registered in the United Kingdom during 1992–2002, 65.6% were caused by P. falciparum, and 27.1% by P. vivax. The higher number corresponds with >50% of persons from Africa, compared with 25% from the Indian subcontinent.
In the United States, cases were usually imported from Central America and Asia by immigrants, as well as by US travelers. However, as in other countries where traditionally P. vivax has been imported, cases acquired in Central and South America and Asia decreased and cases acquired in Africa increased (31). Dorsey et al. found that most patients who imported malaria to the United States had become infected while in Central and South America (38% [35% and 3%, respectively]), followed by West and East Africa (31% [22% and 9%, respectively]), and Asia (29% [Indian subcontinent, 20%; Southeast Asia, 9%]) (32).
In general our findings support those reported in the literature and show that Africa plays a key role in importing malaria in children to industrialized countries where malaria is not endemic. In our study, of all imported malaria cases in children, >70% were acquired in Africa.
Imported malaria depends on the demographics of migrant populations and favored travel destinations of a country’s settled immigrant community, such as the Comorean community in France or the Nigerian community in the United Kingdom. At high risk for malaria are settled immigrants and their children who visit friends and relatives in their country of origin. Many migrants seem to mistakenly believe that they retain their partial immunity against malaria parasites, but immunity usually wanes rapidly (within 6 months) in the absence of exposure to Plasmodium-infected mosquitoes, although some immunologic memory for malaria may exist (8,15,33). In addition, parents of children born and raised in an industrialized country in which malaria is not endemic may mistakenly believe their children have partial immunity (15). Use of chemoprophylaxis is recommended for all children who travel to high-risk malaria-endemic areas (34). Several studies have indicated, however, that correct use of and adherence to chemoprophylaxis is low (13,16,35–38).
Imported malaria in children is a complex problem that faces many challenges, including increasing global migrant and tourist travel; growing proportions of life-threatening falciparum malaria, combined with increasing resistance of malaria parasites to chemoprophylactic drugs; and lack of knowledge about and experience with imported malaria by physicians in industrialized countries where malaria is not endemic, which leads to delays in diagnosis and treatment of children with clinical malaria (32,38). The increasing proportions of P. falciparum cases are of relevance because P. falciparum malaria carries the greatest risk for life-threatening illness. Increasing P. falciparum resistance to antimalarial medication endangers the effectiveness of antimalarial chemoprophylaxis; therefore, standard recommendations for chemoprophylaxis need to be continually updated. Specific research on malaria among children who visit their native countries is warranted. These children are the most likely persons to acquire malaria yet the least likely to use adequate prevention strategies. Culturally sensitive approaches to malaria risk awareness and prevention are urgently needed for schools, the travel industry, and community groups. Local health authorities in communities with large ethnic minorities, particularly of African origin, need to recognize the problem of imported malaria. Some worthwhile community-based programs have been initiated. We conclude that malaria prevention for children should be a task of primary care providers and should be subsidized for low-income travelers to high-risk malaria-endemic areas.
Dr Stäger is a physician whose research interests include travel medicine and malaria in children.
Acknowledgment
We thank Irene Schöneberg and Maria Blaze for their contributions to data compilation and evaluation and Karim Boubaker, Monica Parise, Rob Newman, and Mikio Kimura for helpful suggestions on the manuscript.
References
- World Health Organization. Malaria report 2005 [cited 2008 Feb 12]. Available from http://rbm.who.int/wmr2005
- Sturchler D, Sturchler MP. Global epidemiology of malaria. In: Schlagenhauf P, editor. Travelers’ malaria, 2nd ed. Hamilton (Ontario, Canada): BC Decker; 2008. p. 9–35.
- Muentener P, Schlagenhauf P, Steffen R. Imported malaria (1985–95): trends and perspectives. Bull World Health Organ. 1999;77:560–6.PubMedGoogle Scholar
- Sabatinelli G, Ejov M, Joergensen P. Malaria in the WHO European region (1971–1999). Euro Surveill. 2001;6:61–5.PubMedGoogle Scholar
- Stauffer W, Fischer PR. Diagnosis and treatment of malaria in children. Clin Infect Dis. 2003;37:1340–8. DOIPubMedGoogle Scholar
- United Nations. Trends in total migrant stock: the 2003 revision [cited 2008 Feb 12]. Available from http://www.un.org/esa/population/publications/migstock/2003TrendsMigstock.pdf
- United Nations. World population prospects: the 2002 revision [cited 2008 Feb 12]. Available from http://www.un.org/esa/population/publications/wpp2002/WPP2002-HIGHLIGHTSrev1.pdf
- Schlagenhauf P, Steffen R, Loutan L. Migrants as a major risk group for imported malaria in European countries. J Travel Med. 2003;10:106–7.PubMedGoogle Scholar
- Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA. 2004;291:2856–64. DOIPubMedGoogle Scholar
- Angell SY, Cetron MS. Health disparities among travelers visiting friends and relatives abroad. Ann Intern Med. 2005;142:67–72.PubMedGoogle Scholar
- Health Protection Agency. Illness in England, Wales and Northern Ireland associated with foreign travel: a baseline report to 2002 [cited 2008 Feb 12]. London: The Agency; 2002. Available from http://www.hpa.org.uk/infections/topics_az/travel/pdf/Baseline/full_version.pdf
- Ladhani S, Aibara RJ, Riordan FA, Shingadia D. Imported malaria in children: a review of clinical studies. Lancet Infect Dis. 2007;7:349–57. DOIPubMedGoogle Scholar
- Castéla F, Legros F, Lagardère B. Imported malaria in children in France [in French]. Arch Pediatr. 2003;10:758–65. DOIPubMedGoogle Scholar
- Minodier P, Retornaz K, Kone-Paut I, Garnier JM, Lafay V. Pediatric malaria imported in France [in French]. Arch Pediatr. 2001;8(Suppl 2):266s–8s. DOIPubMedGoogle Scholar
- Brabin BJ, Ganley Y. Imported malaria in children in the UK. Arch Dis Child. 1997;77:76–81. DOIPubMedGoogle Scholar
- López-Vélez R, Huerga H. Imported malaria in children [in Spanish]. An Esp Pediatr. 2000;52:303–4.PubMedGoogle Scholar
- Office for National Statistics. Travel trends, a report on the 2000 International Passenger Survey; 2001 [cited 2008 Feb 12]. Available from http://www.statistics.gov.uk/downloads/theme_transport/TTRENDS2000.pdf
- United Nations World Tourism Organization. Tourism market trends, 2004—Africa. Madrid: The Organization; 2004.
- Legros F, Danis M. Surveillance of malaria in European Union countries. Euro Surveill. 1998;3:45–7.PubMedGoogle Scholar
- Schöneberg I, Krause G, Ammon A, Strobel H, Stark K. Malaria surveillance in Germany 2000/2001–results and experience with a new reporting system [in German]. Gesundheitswesen. 2003;65:263–9. DOIPubMedGoogle Scholar
- Schöneberg I, Stark K, Altmann D, Krause G. Malaria in Germany 1993 to 2003. Data from the Robert Koch Institute on affected groups of people, countries traveled to and treatment [in German]. Dtsch Med Wochenschr. 2005;130:937–41. DOIPubMedGoogle Scholar
- Harvey B. Trends in malaria in Australia, 1991–1997. Commun Dis Intell. 1998;22:247–8.PubMedGoogle Scholar
- Chen LH, Wilson ME, Schlagenhauf P. Prevention of malaria in long-term travelers. JAMA. 2006;296:2234–44. DOIPubMedGoogle Scholar
- Parez N, Delée S, Favier R, Adam M, Quinet B, Grimprel E, Imported malaria in children in 1999. Study of the Armand-Trousseau Hospital in Paris [in French]. Arch Pediatr. 2002;9:371–6. DOIPubMedGoogle Scholar
- Minodier P, Lanza-Silhol F, Piarroux R, Garnier JM, Dumon H, Unal D. Imported paediatric malaria in Marseille [in French]. Arch Pediatr. 1999;6:935–43. DOIPubMedGoogle Scholar
- Eloy O, Bruneel F, Diebold C, Belaid Y, Foucaud P, Charara O, Paediatric imported malaria. Experience of the hospital center of Versailles (1997–2001) [in French]. Ann Biol Clin (Paris). 2003;61:449–53.PubMedGoogle Scholar
- Sabatinelli G, Majori G. Malaria surveillance in Italy: 1986–1996 analysis and 1997 provisional data. Euro Surveill. 1998;3:38–40.PubMedGoogle Scholar
- Romi R, Boccolini D, Majori G. Malaria incidence and mortality in Italy in 1999–2000. Euro Surveill. 2001;6:143–7.PubMedGoogle Scholar
- Williams JP, Chitre M, Sharland M. Increasing Plasmodium falciparum malaria in southwest London: a 25 year observational study. Arch Dis Child. 2002;86:428–30. DOIPubMedGoogle Scholar
- Ladhani S, El Bashir H, Patel VS, Shingadia D. Childhood malaria in East London. Pediatr Infect Dis J. 2003;22:814–9. DOIPubMedGoogle Scholar
- Filler S, Causer LM, Newman RD, Barber AM, Roberts JM, MacArthur J, Malaria surveillance—United States, 2001. MMWR Surveill Summ. 2003;52:1–14.PubMedGoogle Scholar
- Dorsey G, Gandhi M, Oyugi JH, Rosenthal PJ. Difficulties in the prevention, diagnosis, and treatment of imported malaria. Arch Intern Med. 2000;160:2505–10. DOIPubMedGoogle Scholar
- Deloron P, Chougnet C. Is immunity to malaria really short-lived? Parasitol Today. 1992;8:375–8. DOIPubMedGoogle Scholar
- Fischer PR. Travel with infants and children. Infect Dis Clin North Am. 1998;12:355–68. DOIPubMedGoogle Scholar
- Minodier P, Kone-Paut I, Nassur A, Launay F, Jouve JL, Hassid S, Antimosquito precautions and medical chemoprophylaxis in French children with malaria. J Travel Med. 2003;10:318–23.PubMedGoogle Scholar
- Huerga H, López-Vélez R. Imported malaria in immigrant and travelling children in Madrid. Eur J Clin Microbiol Infect Dis. 2001;20:591–3. DOIPubMedGoogle Scholar
- Matteelli A, Colombini P, Gulletta M, Castelli F, Carosi G. Epidemiological features and case management practices of imported malaria in northern Italy 1991–1995. Trop Med Int Health. 1999;4:653–7. DOIPubMedGoogle Scholar
- Viani RM, Bromberg K. Paediatric imported malaria in New York: delayed diagnosis. Clin Pediatr (Phila). 1999;38:333–7. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 15, Number 2—February 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Patricia Schlagenhauf, University of Zürich Centre for Travel Medicine, WHO Collaborating Centre for Travellers’ Health, Hirschengraben 84, CH-8001 Zürich, Switzerland
Top