Volume 15, Number 4—April 2009
Research
Novel Type of Streptococcus pneumoniae Causing Multidrug-Resistant Acute Otitis Media in Children
Abstract
After our recent discovery of a Streptococcus pneumoniae 19A “superbug” (Legacy strain) that is resistant to all Food and Drug Administration–approved antimicrobial drugs for treatment of acute otitis media (AOM) in children, other S. pneumoniae isolates from children with AOM were characterized by multilocus sequence typing (MLST). Among 40 isolates studied, 16 (40%) were serotype 19A, and 9 (23%) were resistant to multiple antimicrobial drugs. Two others had unreported sequence types (STs) that expressed the 19A capsule, and 8 (88%) of the 9 multidrug-resistant strains were serotype 19A, including the Legacy strain with the new ST-2722. In genetic relatedness, ST-2722 belonged to a cluster of reported strains of S. pneumoniae in which all strains had 6 of the same alleles as ST-156. The multidrug-resistant strains related to ST-156 expressed different capsular serotypes: 9V, 14, 11A, 15C, and 19F.
The increasing global emergence and rapid spread of multidrug-resistant Streptococcus pneumoniae is a serious concern (1,2). Clonal dissemination of problematic pneumococcal strains have created clinically important treatment problems (3,4). Pneumococcal resistance to antimicrobial drugs was first reported in the mid-1960s (5,6). Since 1990, drug-resistant isolates of S. pneumoniae have spread rapidly throughout the United States (7). In the early 1990s, high-level resistance to penicillin and other antimicrobial drugs appeared in the United States with a low prevalence (8). Over the past decade, multidrug-resistant clones of S. pneumoniae have rapidly emerged (8–11). Of 90 serotypes, 19A is one of the most common types found in children. It can cause severe disease and easily develop antimicrobial drug resistance (9). The first 19A strain of S. pneumoniae with penicillin resistance was reported in the United States in 1986 (12). Introduction in 2000 of a pneumococcal 7-valent pneumococcal conjugate vaccine (PCV7) in the United States has substantially curtailed pneumococcal infections caused by 7 vaccine strains in children (13–15). However, nonvaccine strains, including drug-resistant strains, are increasingly being identified in patients. Among multidrug-resistant strains, a high proportion is serotype 19A, a strain not included in the vaccine; however, the proportion of invasive pneumococcal diseases caused by serotype 19A has substantially increased (8–11).
We recently discovered and reported a “superbug” strain of S. pneumoniae (Legacy strain) that is resistant to all Food and Drug Administration (FDA)–approved antimicrobial drugs and to 8 non-FDA–approved antimicrobial drugs used to treat acute otitis media (AOM) in children (11). Using molecular epidemiologic methods, particularly multilocus sequence typing (MLST), we characterized the molecular type of multidrug-resistant strains of S. pneumoniae recently isolated from children with AOM, compared the Legacy strain sequence type (ST) 2722 with 67 strains with the closest types in the MLST database, and reported the likely evolution and spread of the ancestor strains of the Legacy strain.
Pneumococcal Isolates, Serotype Analysis, and Antimicrobial Drug–Susceptibility Testing
All pneumococcal bacteria were isolated from children seen at a private pediatric group in suburban Rochester, New York (Legacy Pediatrics). Isolates were obtained from middle ear fluid of children with AOM at 6–36 months of age during 2004–2006. The children received age-appropriate inoculations with PCV7 at 2, 4, 6, and 12–15 months of age. The University of Rochester Institutional Review Board approved the study, and written informed consent was obtained from parents or guardians for the study and for all tympanocentesis procedures. Serotypes of pneumococci were determined by latex agglutination (Pneumotest-Latex; Statens Serum Institute, Copenhagen, Denmark) according to the manufacturer's instructions and confirmed by Quelling reaction. The antimicrobial drug susceptibility of pneumococci was determined as described previously by E-test or microbroth dilution (11).
Multilocus Sequence Typing
The internal fragments of 7 housekeeping genes (aroE [shikimate dehydrogenase], gdh [glucose-6-phosphate dehydrogenase], gki [glucose kinase], recP [transketolase], spi [signal peptidase I], xpt [xanthine phosphoribosyltransferase], and ddl [D-alanine-D-alanine ligase]) were amplified from chromosomal DNA by PCR. Chromosomal DNA was extracted from subculture of S. pneumoniae isolations recovered from middle ear fluid, nasal wash, or nasal swabs. PCR amplification was performed using primer pairs aroE-up, 5′-GCCTTTGAGGCGACAGC-3′ and aroE-dn, 5′-TGCAGTTCA(G/A)AAACAT(A/T)TTCTAA-3′; gdh-up, 5′-ATGGACAAACCAGC (G/A/T/C)AG(C/T)TT-3′ and gdh-dn, 5′-GCTTGAGGTCCCAT(G/A)CT(G/A/T/C) CC-3′; gki-up, 5′-GGCATTGGAATGGGATCACC-3′ and gki-dn, 5′-TCTCCCGCAGCTGACAC-3′; recP-up, 5′-GCCAACTCAGGTCATCCAGG-3′ and recP-dn, 5′-TGCAACCGTAGCATTGTAAC-3′; and spi-up, 5′-TTATTCCTCCTGATTCTGTC-3′ and spi-dn, 5′-GTGATTGGCCAGAAGCGGAA-3′. PCR conditions were as follows: initial denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 30 s; annealing at 50°C–55°C for 30 s; and extension at 72°C for 30 s. The amplified DNA fragments were purified by using QIAquick PCR Purification Kit (QIAGEN, Valencia, CA, USA) and were sequenced in each direction by using the same primers used for amplification and by using the BigDye Terminator v3.1 Cycle Sequencing Kit on an Applied Biosystems Prism 377 automated sequencer (Applied Biosystems, Foster City, CA, USA).
The sequences at each of the 7 loci were then compared with the sequences of all of the known alleles at those loci in the database at the pneumococcal MLST website (http://spneumoniae.mlst.net). The sequences identical to a known sequence were assigned the same allele number, and nonidentities to any known allele sequences were assigned new allele numbers. The allele at each of the 7 loci defines the allelic profile of each strain, as well as its ST. New allelic number or new ST number was assigned by a curator of the pneumococcal MLST database. The relatedness of isolates and known similar strains in the database was determined by constructing a neighbor-joining tree using a program online, Draw Tree Using Own MLST Data, found at the pneumococcal MLST website.
Among 40 S. pneumoniae isolates recovered from middle ear fluid of children with AOM, 16 (40%) were serotype 19A. Serotype 19A was the most common serotype isolated in the study during 2004–2006.
Antimicrobial drug susceptibility testing was performed on the serotype 19A isolates. Eight (50%) of the 16 isolates of 19A were highly penicillin resistant (MIC >2.0 µg/mL), and all 8 were also multidrug resistant.
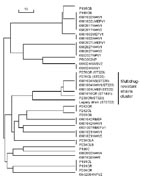
MLST of these16 S. pneumoniae isolates with serotype 19A showed that 6 (38%) were ST-320; 3 (19%) were ST-199; 2 (13%) were unreported STs now assigned ST- 2722 and ST-2704; 2 (13%) were ST-1673; and 1 (6%) each of the remaining 3 was ST-1451, ST-2265, and ST-63. Among the 8 multidrug-resistant isolates, 5 (63%) were ST-320, 1 (13%) was ST-1451, and 1 (13%) was the newly assigned ST-2722 (Table 1, Figure 1). The genetic distance of the Legacy strain is closest to 7 other multidrug-resistant strains with ST-320 and ST-1451 in our group of 40 recent isolates.
To determine the evolutionary relationship of the Legacy strain (ST-2722) to other strains, we constructed a neighbor-joining tree. ST-2722 is assumed to have genetic relatedness to another ST in the database when an ST has >5 of the same loci as ST-2722 (Figure 2). ST-2722 belonged to a cluster in which all strains have 6 of 7 same loci. This cluster consisted of 59 (88%) strains with molecular type ST-156 and 1 strain each with ST-2722, ST-2128, ST-1227, ST-2616, ST-1893, ST-334, ST-1556, ST-2684, and ST-1697. The major dissimilarity of strains in this cluster was on loci recP (Table 2, Figure 3). The recP gene had 3 major variable sites at the 10th, 121st, and 368th bp among strains. For instance, the DNA at the 10th, 121st, and 368th bp are T, C, T, respectively, in ST-2722; C, T, T in ST-156; and C, T, C in ST-2128 (Figure 3).
Forty-three (64%) of the 67 strains related to ST-2722 were penicillin resistant, and 28 (42%) of these strains were resistant to at least 1 other antimicrobial drug. Thirty-four (51%) of the 67 strains related to ST-2722 serotype 19A strains were serotype 9V; 18 (27%) were serotype 14; and 5 (7%) were serotype 11A. Among the 43 antimicrobial drug–resistant strains in this cluster, 24 (56%) were serotype 9V, and 14 (33%) were serotype 14; others were serotype 11, 15C, and 19F.
We described the molecular and capsular types of pneumococci causing AOM in children tested in Rochester, New York, during 2004–2006. Serotype 19A, a relatively new molecular type, accounted for 40% of the isolates and has emerged as the major serotype causing ear infections in children. Eight different molecular STs expressed the 19A capsule; most of the strains were multidrug resistant. Among the 40 strains studied, 5% were new molecular STs. In addition, we analyzed the genetic relatedness of the strains to other previously described strains of serotype 19A. We traced the genetic origin of ST-2722 to a strain first identified in 1988 in Spain as ST-156, which expressed capsular serotype 9V (16). Over nearly 20 years before ST-2722 emerged, variants of this original ST-156 strain were identified in 18 countries, with 8 different STs and 13 different sequence/capsular type combinations (according to records in S. pneumoniae database).
ST-2722 (Legacy strain) appears to have resulted from a mutation identified in the recP gene that coincided with acquisition of multidrug resistance, including a ceftriaxone MIC of 6.0 µg/mL, and acquisition of capsular DNA associated with the 19A serotype. Because of genetic plasticity, different capsular genes of S. pneumoniae may transfer through DNA-mediated genetic recombination (17–19). The multidrug-resistant epidemic type 23F Spanish/USA clone has expressed capsular types 3, 9N, 14, and 19F. Capsular transformation may equip multidrug-resistant strains with highly virulent blood invasive phenotypes (18).
Many studies have documented the impact of the PCV7 on pneumococcal diseases. Introduction of PCV7 containing serotype 4, 6B, 9V, 14, 18C, 19F, and 23F has dramatically decreased the rate of carriage and disease caused by these vaccine serotypes. However, the proportion of invasive pneumococcal diseases caused by nonvaccine serotypes has increased substantially in recent years, and multiresistant strains have rapidly emerged (16,20–23). In addition, PCV7 offers moderate protection against AOM (24). The lowest level of protective activity provided by PCV7 was against serotype 19F, and 19F polysaccharide antigen provided less cross-protection for disease caused by related serotypes such as 19A (24,25). An increase in the rate of middle ear infections with 19A strains and other strains expressing capsular types not contained in PCV7 also has been reported (11). These developments encourage further ongoing epidemiologic surveillance. Emergence of new 19A strains may represent a successful vaccine escape mechanism used by PCV7-targeted clones, and antimicrobial drug nonsusceptibility provides an additional survival advantage that could help these organisms spread further.
By MLST analysis and capsular typing, we found that multiple STs expressing capsular type 19A have emerged as the most important otopathogens of children. We also found a new strain of S. pneumoniae (ST-2722), expressing a 19A capsule that is resistant to all FDA-approved antimicrobial drugs. ST-2722 has a genetic relatedness close to ST-156 reported to the S. pneumoniae MLST database from 18 countries. ST-2722 was multiresistant to antimicrobial drugs but had an MIC to ceftriaxone of 6.0 µg/mL. A study by Pelton et al. showed that multidrug-resistant 19A strains with ST-320 had an MIC to ceftriaxone of 8.0 µg/mL (10). The clinical importance of such strains is potentially high because empiric treatment of suspected and even proven pneumococcal infections typically relies on the efficacy of ceftriaxone.
Dr. Xu is an instructor of microbiology and immunology at University of Rochester Medical Center, Rochester, New York. His primary research interests include bacterial pathogenesis and vaccine development.
Acknowledgments
We gratefully acknowledge the staff of Legacy Pediatrics for providing S. pneumoniae isolates. We also express appreciation to Cynthia Bishop, the curator for the S. pneumoniae MLST database (http://spneumoniae.mlst.net).
This work was supported by the Thrasher Research Fund (Salt Lake City, UT, USA) award #02823-2 to M.E.P. and by an investigator-initiated research grant from Wyeth Pharmaceuticals (Collegeville, PA, USA) to M.E.P. Q.X. and M.Z. also were supported by the National Institute on Deafness and Other Communication Disorders research grant DC05845 to M.Z.
References
- Rahman M, Hossain S, Shoma S, Rashid H, Hel Baqui A, van der Linden M, Emergence of a unique multiply-antibiotic-resistant Streptococcus pneumoniae serotype 7B clone in Dhaka, Bangladesh. J Clin Microbiol. 2006;44:4625–7. Epub 2006 Sep 27. DOIPubMedGoogle Scholar
- Vilhelmsson SE, Tomasz A, Kristinsson KG. Molecular evolution in a multidrug-resistant lineage of Streptococcus pneumoniae: emergence of strains belonging to the serotype 6B Icelandic clone that lost antibiotic resistance traits. J Clin Microbiol. 2000;38:1375–81.PubMedGoogle Scholar
- Odenholt I, Gustafsson I, Lowdin E, Cars O. Suboptimal antibiotic dosage as a risk factor for selection of penicillin-resistant Streptococcus pneumoniae: in vitro kinetic model. Antimicrob Agents Chemother. 2003;47:518–23. DOIPubMedGoogle Scholar
- Bean DC, Klena JD. Characterization of major clones of antibiotic-resistant Streptococcus pneumoniae in New Zealand by multilocus sequence typing. J Antimicrob Chemother. 2005;55:375–8. Epub 2005 Jan 28. DOIPubMedGoogle Scholar
- Kislak JW, Razavi LM, Daly AK, Finland M. Susceptibility of pneumococci to nine antibiotics. Am J Med Sci. 1965;250:261–8.PubMedGoogle Scholar
- Dagan R, Givon-Lavi N, Zamir O, Fraser D. Effect of a nonavalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr Infect Dis J. 2003;22:532–40. DOIPubMedGoogle Scholar
- Samore MH, Magill MK, Alder SC, Severina E, Morrison-De Boer L, Lyon JL, High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics. 2001;108:856–65. DOIPubMedGoogle Scholar
- Mera RM, Miller LA, Daniels JJ, Weil JG, White AR. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander Project. Diagn Microbiol Infect Dis. 2005;51:195–200. DOIPubMedGoogle Scholar
- Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Curr Opin Infect Dis. 2003;16:271–7.PubMedGoogle Scholar
- Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. DOIPubMedGoogle Scholar
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772–8. DOIPubMedGoogle Scholar
- Simberkoff MS, Lukaszewski M, Cross A, Al-Ibrahim M, Baltch AL, Smith RP, Antibiotic-resistant isolates of Streptococcus pneumoniae from clinical specimens: a cluster of serotype 19A organisms in Brooklyn, New York. J Infect Dis. 1986;153:78–82.PubMedGoogle Scholar
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. DOIPubMedGoogle Scholar
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–502. DOIPubMedGoogle Scholar
- Poehling KA, Szilagyi PG, Grijalva CG, Martin SW, LaFleur B, Mitchel E, Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–15. DOIPubMedGoogle Scholar
- Zhou J, Enright MC, Spratt BG. Identification of the major Spanish clones of penicillin-resistant pneumococci via the Internet using multilocus sequence typing. J Clin Microbiol. 2000;38:977–86.PubMedGoogle Scholar
- Lopez R, Garcia E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev. 2004;28:553–80. DOIPubMedGoogle Scholar
- Nesin M, Ramirez M, Tomasz A. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis. 1998;177:707–13.PubMedGoogle Scholar
- Trzcinski K, Thompson CM, Lipsitch M. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl Environ Microbiol. 2003;69:7364–70. DOIPubMedGoogle Scholar
- Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. DOIPubMedGoogle Scholar
- Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005;192:1988–95. Epub 2005 Nov 1. DOIPubMedGoogle Scholar
- McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med. 2003;9:424–30. Epub 2003 Mar 10. DOIPubMedGoogle Scholar
- Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–60.PubMedGoogle Scholar
- Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006). Vaccine. 2007;25:2194–212. DOIPubMedGoogle Scholar
- Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003;37:1155–64. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 15, Number 4—April 2009
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Mingtao Zeng, Department of Microbiology and Immunology, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box 672, Rochester, NY 14642
Top