Volume 16, Number 3—March 2010
Dispatch
Influenza A Pandemic (H1N1) 2009 Virus Infection in Domestic Cat
Abstract
Influenza A pandemic (H1N1) 2009 virus continues to rapidly spread worldwide. In 2009, pandemic (H1N1) 2009 infection in a domestic cat from Iowa was diagnosed by a novel PCR assay that distinguishes between Eurasian and North American pandemic (H1N1) 2009 virus matrix genes. Human-to-cat transmission is presumed.
Influenza viruses are typically host specific; aquatic birds are considered the primary reservoir. However, interspecies transmission does occur (1–9) and occasionally leads to novel host-adapted strains. Interspecies transmission of influenza virus has been a public health concern because of the possibility that, through reassortment, a novel strain with zoonotic potential could emerge. The recent infection of dogs with equine influenza virus (H3N8) (2) and of swine with human influenza virus (H1N2) (4) are particularly intriguing because the former resulted in influenza becoming endemic in dogs and the latter resulted in a documented reassortment event between human and swine influenza viruses. Such concern has escalated with the recent emergence of the novel quadruple-reassorted influenza virus (H1N1) [pandemic (H1N1) 2009] in humans (10). Although infection and transmission of the virus have occurred primarily among humans, occasional transmission from infected persons to susceptible animals (e.g., swine, turkeys, ferrets) has been documented (11). The likelihood of pandemic (H1N1) 2009 infection of domestic pets has been considered less likely (www.cdc.gov/h1n1flu/qa.htm, www.avma.org/public_health/influenza/new_virus/default.asp, www.usda.gov/wps/portal/?navid=USDA_H1N1); however, we report a confirmed case of pandemic (H1N1) 2009 virus infection in a domestic cat that had been in contact with persons who had recently experienced influenza-like illness.
A 13-year-old, castrated male, domestic cat that lived indoors in a single-cat household was brought to the Iowa State University Lloyd Veterinary Medical Center because of depression, inappetance, and respiratory signs of 4 days’ duration. The cat was gregarious and interacted closely with family members in the household. The family members noted that the cat was reluctant to lie in lateral recumbency and instead rested in sternal recumbency with neck extended, which was indicative of dyspnea. The cat’s vaccination status was up to date. Before the onset of clinical signs in the cat, 2 of the 3 family members had experienced an undiagnosed influenza-like illness—an upper respiratory tract infection characterized by fever, coughing, and myalgia—that lasted 3 days. Onset of the cat’s clinical signs was noted 6 and 4 days after onset of illness for the first and second family members, respectively.
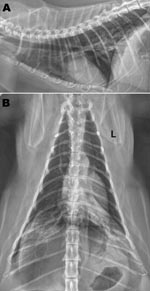
At the time of examination, the cat had bilateral adventitial lung sounds (wheezes), was afebrile, and was clinically dehydrated. Radiographs of the thorax showed a bilateral caudodorsal alveolar pattern (Figure). Cytologic and microbiologic examination of bronchoalveolar lavage (BAL) fluid showed foamy macrophages (65%), nondegenerate neutrophils (25%), and small lymphocytes (10%). Clinicopathologic findings suggested a moderate, predominantly macrophagic, mixed inflammatory process. Standard microbial culture of BAL aliquots yielded no substantial growth of aerobic or anaerobic bacteria. Radiographic and cytologic findings were inconsistent with bacterial or parasitic pneumonia and not supportive of allergic airway disease. A viral cause was considered most likely; however, the cat was given amoxicillin with clavulanate (125 mg orally 2×/day) to reduce the possibility of secondary bacterial pneumonia. Notable findings from laboratory testing (complete blood count, serum biochemistry, urinalysis, and total thyroxine measurement) were moderate leukopenia characterized by a moderate lymphopenia, modest hemoconcentration, and a slightly elevated thyroxine level. Lymphopenia was consistent with acute viral infection.
PCR testing (Feline URD Panel; Idexx Laboratories, Westbrook, ME, USA) of a BAL sample showed negative results for Chlamydophila felis, feline calicivirus, feline herpesvirus-1, Bordetella bronchiseptica, and Mycoplasma felis. Results of feline immunodeficiency virus (antibody) and feline leukopenia virus (antigen) testing (Idexx SNAP FIV/FeLV Combo Test; Idexx Laboratories) were also negative, ruling out the potential that viral-induced immunosuppression was a concurrent factor. For the following reasons we included pandemic (H1N1) 2009 on our list of differential diagnoses: recent history of respiratory disease in household family members, known widespread community prevalence of pandemic (H1N1) 2009 influenza in humans, paucity of common viral infections causing infectious caudodorsal alveolar pneumonia in adult cats, and documented susceptibility of felids to avian influenza (H5N1) (12,13). We therefore submitted a BAL sample to the Iowa State University Veterinary Diagnostic Laboratory for molecular screening and typing for influenza A and the pandemic (H1N1) 2009 virus.
RNA was obtained from the BAL fluid by using the MagMAX Viral RNA Isolation Kit (Applied Biosystems, Austin, TX, USA) and a semiautomated magnetic particle processor (Kingfisher 96; Thermo Electron Corp., Woodstock, GA, USA) according to manufacturer’s recommendations. Molecular testing used a real-time reverse transcription–PCR (rRT-PCR) influenza A screening assay specific for the nucleoprotein gene. Preliminary differentiation of pandemic (H1N1) 2009 virus from other H1 or H3 types of influenza A was performed by using an in-house rRT-PCR assay that distinguishes between pandemic (H1N1) 2009 [Eurasian matrix (10)] and endemic (to North America) swine H1N1 influenza viruses (North American matrix). Sequences of primers and probes are summarized in Table 1. PCRs were conducted by using the AgPath-ID Multiplex One-Step RT-PCR Kit (Ambion/Applied Biosystems) according to manufacturer’s recommendations; 10 units of Multiscribe Reverse Transcriptase (Applied Biosystems) were added per reaction. Thermocycling was performed by using the Applied Biosystems 7500 Fast Real-Time PCR System according to manufacturer’s recommendations.
PCR testing showed the BAL sample to be positive for influenza A virus (nucleoprotein gene), and the virus was determined to contain the matrix (M) gene of the pandemic (H1N1) 2009 virus strain. A BAL sample was submitted to the US Department of Agriculture National Veterinary Services Laboratories (Ames, IA, USA) for confirmatory testing. rRT-PCR confirmed that the BAL sample was positive for the M gene of influenza A virus and the neuraminidase (N) gene of pandemic (H1N1) 2009 virus. Sequences of primers and probes are summarized in Table 2. A cytolytic virus was isolated by using MDCK cells (8) and was designated as A/feline/IA/NVSL026991/2009. PCR testing of the isolate for influenza A virus (M gene) and N1 gene of pandemic (H1N1) 2009 showed positive results. Sequence analyses for hemagglutinin (HA), N, and M genes confirmed that the virus was pandemic (H1N1) 2009 virus (GenBank accession nos. GU332630 (for HA), GU332632 (for NA), and GU332631 (for M). Nucleotide homologies with the first US human pandemic (H1N1) 2009 isolate (A/CA/04/2009) were 99.4%, 99.4%, and 99.8% for the HA, NA, and M genes, respectively.
The cat was discharged from the medical center after diagnostic testing and correction of dehydration. A veterinarian (B.A.S.) visited the home to monitor the cat’s clinical status and administer subcutaneous fluids (120–160 mL) until the cat’s appetite improved; adventitial lung sounds resolved within 3 days. Reassessment 1 week later showed marked improvement of clinical signs but only modest improvement of the lymphopenia and radiographic findings.
Because the cat was from a single-animal household and remained indoors, he was presumably infected through contact with the family members. Attempts to retrospectively confirm pandemic (H1N1) 2009 infection in the family members have been unsuccessful, but additional testing of archived biologic samples is being conducted. Although more surveillance and studies are needed to determine susceptibility of companion animals to the pandemic (H1N1) 2009 virus, possible reverse zoonotic transmission (humans to animals) remains a concern. Indeed, cases in a domestic dog and other felids have been confirmed (11) (www.cdc.gov/h1n1flu/qa.htm, www.avma.org/public_health/influenza/new_virus/default.asp, www.usda.gov/wps/portal/?navid=USDA_H1N1). Implications of pandemic (H1N1) 2009 virus infection in companion animals are 1) apparent human-to-animal transmission; 2) broader host range for the virus; 3) potential endemic establishment of influenza in companion animals; 4) possible transmission of influenza from companion animals to other species, including humans; and 5) the need to reevaluate companion animals as potential reservoirs or intermediate hosts for reassortment of influenza virus. This case emphasizes the need for close monitoring for interspecies transmission of influenza virus and reinforces the need for collaboration among many disciplines, a cornerstone of the One Health Initiative (www.onehealthinitiative.com).
Dr Sponseller is an assistant professor in the Departments of Veterinary Clinical Sciences and Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University. His research focuses on viral pathogens of domestic animals and acquisition of pulmonary immunocompetency.
Acknowledgments
We thank the family members in the cat’s household for their cooperation and Sarah Abate, Wendy Stensland, and Leslie Bower for technical assistance.
This study was supported by the Iowa State University Office of the Vice President for Research and Economic Development, the Iowa Healthy Livestock Initiative Research Grant, and the Center for Advanced Host Defenses, Immunobiotics and Translational Comparative Medicine.
References
- Choi YK, Lee JH, Erickson G, Goyal SM, Joo HS, Webster RG, H3N2 influenza virus transmission from swine to turkeys, United States. Emerg Infect Dis. 2004;10:2156–60.PubMedGoogle Scholar
- Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, Transmission of equine influenza virus to dogs. Science. 2005;310:482–5. DOIPubMedGoogle Scholar
- Gagnon CA, Spearman G, Hamel A, Godson DL, Fortin A, Fontaine G, Characterization of a Canadian mink H3N2 influenza A virus isolate genetically related to triple reassortant swine influenza virus. J Clin Microbiol. 2009;47:796–9. DOIPubMedGoogle Scholar
- Karasin AI, Carman S, Olsen CW. Identification of human H1N2 and human–swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005). J Clin Microbiol. 2006;44:1123–6. DOIPubMedGoogle Scholar
- Patterson AR, Cooper VL, Yoon KJ, Janke BH, Gauger PC. Naturally occurring influenza virus infection in a ferret (Mustela putorius furo) colony. J Vet Diagn Invest. 2009;21:527–30.PubMedGoogle Scholar
- Song D, Kang B, Lee C, Jung K, Ha G, Kang D, Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008;14:741–6. DOIPubMedGoogle Scholar
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis. 2006;12:681–3.PubMedGoogle Scholar
- Vincent AL, Swenson SL, Lager KM, Gauger PC, Loiacono C, Zhang Y. Characterization of an influenza A virus isolated from pigs during an outbreak of respiratory disease in swine and people during a county fair in the United States. Vet Microbiol. 2009;137:51–9. DOIPubMedGoogle Scholar
- Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol. 1999;73:8851–6.PubMedGoogle Scholar
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. DOIPubMedGoogle Scholar
- United States Department of Agriculture. 2009 Pandemic H1N1 influenza presumptive and confirmed results [cited 2009 Dec 23]. http://www.usda.gov/documents/FINAL_RESULTS_2009_PANDEMIC_H1N1_INFLUENZA_CHT.pdf
- Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Avian H5N1 influenza in cats. Science. 2004;306:241. DOIPubMedGoogle Scholar
- Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RA, Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. [quiz 364]. Am J Pathol. 2006;168:176–83. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 16, Number 3—March 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Brett A. Sponseller, 2134 College of Veterinary Medicine, Iowa State University, 1600 S 16th St, Ames, IA 50011-1248, USA
Top