Volume 16, Number 8—August 2010
Dispatch
Amblyomma imitator Ticks as Vectors of Rickettsia rickettsii, Mexico
Abstract
Real-time PCR of Amblyomma imitator tick egg masses obtained in Nuevo Leon State, Mexico, identified a Rickettsia species. Sequence analyses of 17-kD common antigen and outer membrane protein A and B gene fragments showed to it to be R. rickettsii, which suggested a potential new vector for this bacterium.
Rickettsia rickettsii is a gram-negative, obligate, intracellular bacterium and the cause of Rocky Mountain spotted fever (RMSF). In Mexico, its transmission has been attributed to Rhipicephalus sanguineus and Amblyomma cajennense ticks.
Amblyomma imitator has close affinity with A. cajennense and was formerly confused with this species. These ticks’ distributional range extends from southern Texas, southward through Mexico (where they are widely sympatric with A. cajennense ticks) into Central America (1). In this study, we isolated and characterized R. rickettsii from A. imitator from Mexico by using molecular methods.
Males, females, and nymphs of 5 tick species (A. imitator, Rhipicephalus microplus, Dermacentor variabilis, Rh. sanguineus, and Ixodes species) were collected in Nuevo Leon State, Mexico (24°50′N, 100°4′W) in 2007 (females, males, and nymphs) by using dry ice traps (1). Ticks were identified by using morphologic keys as defined by Kohls (2) and Keirans and Durden (3) and maintained in the laboratory at the University of Texas Medical Branch (Galveston, TX, USA) according to methods described by Brossard and Wikel (4). The colonies were maintained at 22°C, under a 14-hour light/10-hour dark photoperiod. Ticks were held in 16-mL glass vials (Wheaton Glass, Millville, NJ, USA) with a mesh top over a supersaturated solution of potassium nitrate. Larvae and nymphs obtained blood meals from mice, and adults were fed on pathogen-free rabbits. Only the A. imitator colony was successfully established.
Real-time PCR analysis of eggs laid by 1 full generation of laboratory-reared females identified rickettsial DNA in 2 egg masses. DNA from 5 egg masses laid by different females was extracted by using a DNeasy Kit (QIAGEN, Valencia, CA, USA). Real-time PCR was performed by using Rickettsia spp.–specific primers CS5A and CS6 (5) for amplification of a 150-bp fragment of the citrate synthase gene in an iCycler thermocycler (Bio-Rad, Hercules, CA, USA) as described (5). R. australis DNA and water served as the positive and negative controls, respectively, and serial dilutions of a plasmid that contained the R. prowazekii citrate synthase gene were used as standards.
We selected egg masses for isolation of the rickettsial agent in Vero cells by using shell vials as described (6). The shell vials were incubated at 34°C and monitored daily by Diff-Quik staining (Dade International Inc., Miami, FL, USA) for rickettsiae. Slides that contained >4 rickettsiae were considered positive. The monolayer from the corresponding shell vial was removed manually, placed in a T-25 flask containing Vero cells (in Dulbecco minimal essential medium containing 3% heat-inactivated bovine calf serum). Samples were then placed in 150-cm2 flasks containing Vero cells for propagation of the agent.
For characterization of isolates, partial sequences of Rickettsia-specific genes were amplified and analyzed. Nested PCR was performed by using primers 17K3 and 17K5 (5) for the first reaction and 17KD1 and 17kD2 (7) for the second reaction of amplification of 17-kD common antigen (htrA) gene fragments. For amplification of an outer membrane protein B (ompB) gene fragment, primers 120-M59 and 120–807 were used (8). Seminested PCR with primers 120-M59 and 120–807 for the first reaction and 120–607 and 120–807 for the second reaction was performed for 1 of the samples (8). An ompA fragment was amplified by using primers Rr190.70F and Rr190.602R (9). For 1 of the samples, a seminested PCR was necessary; primers Rr190.70F and Rr190.701R (10) were used for the first reaction and Rr190.70F and Rr190.602R for the second reaction.
Fragments were cloned into pCR4-TOPO (Invitrogen, Carlsbad, CA, USA), and plasmids from selected clones were sequenced >3× by using universal primers M13F and M13R. Nucleotide sequences were edited with SeqMan (www.dnastar.com/t-sub-products-lasergene-seqmanpro.aspx) and used for BLAST analysis (11). Amplification of the ompB fragment was achieved for only 1 of the samples. This finding was probably the result of a mutation at the site of primer annealing because amplification of another region of the gene was possible.
Analysis of sequences obtained from the htrA gene fragments (434 bp) amplified from both samples showed 99% identity with spotted fever group Rickettsia sequences, including the R. rickettsii sequence from a fatal case of RMSF in southwestern Mexico, Yucatan State (GenBank accession no. DQ176856.1) (12), with only a 1-nt difference. Analysis of the partial sequence of ompB (856 bp) and ompA (533 bp) genes showed 100% identity with R. rickettsii Sheila Smith strain (CP000848.1). Sequences obtained in this study were submitted to GenBank under accession nos. GU723476 and GU723477 for htrA fragments, GU723478 and GU723479 for ompA fragments, and GU723475 for the ompB fragment.
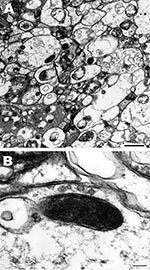
For detection of rickettsiae in A. imitator ticks, salivary glands, midguts, and ovaries were dissected from unfed adult ticks and fixed in modified Ito fixative (13), postfixed in 1% osmium tetroxide for 1 h, stained en bloc with 2% aqueous uranyl acetate for 20 min at 60°C, dehydrated in ethanol, and embedded in epoxy resin (Poly/Bed 812). Ultrathin sections were cut with an Ultracut S Ultramicrotome (Reichert, Vienna, Austria), placed on copper grids, and stained with lead citrate. Ticks were tested for Rickettsia spp. by nested PCR with primers 17K3/17K5 and 17KD1/17KD2, and sections of organs from PCR-positive samples were examined in a Philips CM-100 electron microscope (FEI, Hillsboro, OR, USA) at 60 kV.
Single rickettsial cells were found in highly vacuolated cytoplasm of midgut epithelial cells of 1 male tick (no. 5) (Figure, panel A). Cells had typical ultrastructure for gram-negative bacteria and were surrounded by 2 trilaminar membranes (Figure, panel B): an inner cytoplasmic membrane and an outer cell wall membrane. Their size was 0.6 × 0.2 μm. Rickettsial organisms were not visualized in either salivary gland or ovaries probably because of a low number of rickettsiae, as suggested by the fact that detection of rickettsial DNA by PCR required a nested PCR.
This study demonstrated that R. rickettsii was present in egg masses of A. imitator ticks. Because eggs were laid by field-collected, unfed, adult ticks that were fed in the laboratory on pathogen-free rabbits for 1 full generation, R. rickettsii in eggs documents their transovarial transmission by naturally infected ticks and suggests a role for this tick species in the maintenance of R. rickettsii in nature.
Because hosts of A. imitator ticks are various species of birds and mammals (3), the notable finding of this study is the potential participation of A. imitator ticks in a zoonotic cycle of R. rickettsii. According to Kohls (2), RMSF transmission studies performed with supposed A. cajennense ticks by Parker et al. (14) were actually performed with A. imitator ticks, which suggests that this species of tick could be a vector of R. rickettsii. On the basis of the results of our study and the aggressive nature of the tick for humans, we suggest that A. imitator ticks are a potential vector or at least involved in maintenance of R. rickettsii in nature in Mexico.
At the time of this research, Dr Oliveira was a Ph.D candidiate at the Federal University of Vicosa, Vicosa, Brazil. She is currently a research scientist at the Federal University of Vicosa. Her research interests are the biochemistry and molecular biology of ticks and rickettsiae.
Acknowledgment
This study was supported by Fogarty International Center (D43 TW00903) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais/Brazil (APQ-0950-01/07). K.A.O. was supported by a scholarship from Coordenação de Aperfeiçoamento de Passoal de Nival Superior/Brazil and Conselho Nacional de Desenvolvimento Científico e Tecnológico/Brazil (202151/2007-7).
References
- Pinter A, Medina-Sanchez A, Bouyer DH, Teel PD, Fernandes-Salas I, Walker DH. Preliminary data of Amblyomma imitator Kohls, 1958 (Acari:Ixodidae) biology study. In: Abstracts of the VI International Conference on Ticks and Tick-borne Pathogens; Buenos Aires; 2008 Sep 21–26. Abstract P190.
- Kohls GM. Amblyomma imitator, a new species of tick from Texas and Mexico, and remarks on the synonymy of A. cajennense (Fabricius) (Acarina-Ixodidae). J Parasitol. 1958;44:430–3. DOIPubMedGoogle Scholar
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol. 1998;35:489–95.PubMedGoogle Scholar
- Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129(Suppl):S161–76. DOIPubMedGoogle Scholar
- Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride J, Pinter A, Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol. 2004;42:90–8. DOIPubMedGoogle Scholar
- Kelly PJ, Raoult D, Mason PR. Isolation of spotted fever group rickettsiae from triturated ticks using a modification of the centrifugation vial technique. Trans R Soc Trop Med Hyg. 1991;85:397–8. DOIPubMedGoogle Scholar
- Webb L, Mitchell C, Malloy DC, Dasch GA, Azad AF. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol. 1990;28:530–4.PubMedGoogle Scholar
- Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer membrane protein rOmpB (ompB). Int J Syst Evol Microbiol. 2000;50:1449–55.PubMedGoogle Scholar
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89.PubMedGoogle Scholar
- Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein OmpA. J Clin Microbiol. 1996;34:2058–65.PubMedGoogle Scholar
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.PubMedGoogle Scholar
- Zavala-Castro JE, Zavala-Velázquez JE, Walker DH, Arcila EER, Laviada-Molina H, Olano JP, Fatal human infection with Rickettsia rickettsii, Yucatán, Mexico. Emerg Infect Dis. 2006;12:672–4.PubMedGoogle Scholar
- Ito S, Rikihisa Y. Techniques for electron microscopy of rickettsiae. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and rickettsial diseases. New York: Academic Press; 1981. p. 213–27.
- Parker RR, Philip CB, Jellison WL. Rocky Mountain spotted fever. Potentialities of tick transmission in relation to geographical occurrence in the United States. Am J Trop Med. 1933;13:341–79.
Figure
Cite This Article1Current affiliation: Federal University of Vicosa, Vicosa, Brazil.
2Current affiliation: Superintendency of Control of Endemic Diseases, São Paulo, Brazil.
3Current affiliation: Hospital and Clinic Oca, Monterrey, Nuevo Leon, Mexico.
4Current affiliation: Texas A&M University, College Station, Texas, USA.
5Current affiliation: Federal University of Ouro Preto, Ouro Preto, Brazil.
Table of Contents – Volume 16, Number 8—August 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Donald H. Bouyer, Department of Pathology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0609, USA
Top