Volume 17, Number 2—February 2011
Dispatch
Pandemic (H1N1) 2009–associated Pneumonia in Children, Japan
Abstract
To describe clinical aspects of pandemic (H1N1) 2009 virus–associated pneumonia in children, we studied 80 such children, including 17 (21%) with complications, who were admitted to 5 hospitals in Japan during August–November 2009 after a mean of 2.9 symptomatic days. All enrolled patients recovered (median hospitalization 6 days). Timely access to hospitals may have contributed to favorable outcomes.
We describe the clinical aspects of pandemic (H1N1) 2009 virus infection in children who developed spontaneous pneumomediastinum (1) or plastic bronchitis (2). In Mexico, 18 persons, including 5 children, had pandemic (H1N1) 2009–associated pneumonia (3). However, active surveillance to collect data on pneumonia cases among children infected with pandemic (H1N1) 2009 virus has not been conducted in Japan.
Active procurement of specimens from pediatric inpatients with pandemic (H1N1) 2009–associated pneumonia was organized by the Laboratory of Molecular Epidemiology for Infectious Agents at Kitasato University. Clinical data and respiratory specimens were provided by pediatric departments at 5 institutions during August 9–November 6, 2009. Pandemic (H1N1) 2009–associated pneumonia was diagnosed from influenza-like illnesses associated with infiltrates on chest radiographs and laboratory-confirmed pandemic (H1N1) 2009 virus (3). Each patient’s pediatrician informed us of any major complication that followed the pneumonia.
First, patients were divided into 2 groups: those who had and did not have complications. The group having no complications then was divided into 2 age-defined subgroups (cutoff, 6 years). Each subgroup was further divided into subgroups: hospital admission 1–3 days after symptom onset or admission >4 days after symptom onset. Information about clinical features; routine laboratory findings at hospital admission; and if available, serum immunoglobulin E concentration was obtained from patients’ medical charts. Tachypnea was defined by using criteria in Japanese guidelines adopted in 2007 for managing respiratory infectious diseases (4) in children. Chest radiographic findings taken at time of hospital admission were classified by extent of pulmonary infiltrates (localized vs. diffuse) and infiltrate distribution (bilateral vs. unilateral; upper, middle, or lower lung field) (4).
Nasopharyngeal swabs (n = 79) or an endotracheal aspirate were sent to the laboratory for microbiologic identification. Pandemic (H1N1) 2009 virus in specimens was determined by real-time reverse transcription–PCR (RT-PCR) (1,2). Additionally, comprehensive real-time RT-PCR was performed to confirm respiratory co-infection with any of 12 viruses (5). Multiplex real-time PCR also was performed to detect 6 respiratory bacteria (6).
Patient demographic characteristics, symptoms, physical findings, treatments, and clinical courses were compared between groups with and without complications by using the χ2 test. Neutrophil and lymphocyte counts were analyzed by using box-and-whisker plots. A p value <0.05 indicated a significant difference between patient groups.
The study comprised 80 pediatric inpatients who received treatment at 5 medical institutions for pandemic (H1N1) 2009–associated pneumonia over a 3-month period. Family members were informed about the purpose of the study, and children’s parents provided informed consent.
We compared patients by presence or absence of complications (Table 1). Complications included pleural effusion (5 patients), pneumomediastinum (6), atelectasis (6), myositis (2), and plastic bronchitis (1). No patients had organ dysfunction or encephalopathy.
The median age of pneumonia patients was 7 years; 57 (71%) were male; 26 (33%) had asthma, 4 (5%) had atopic dermatitis without asthma, and 1 (1%) had DiGeorge syndrome. Forty-nine (61%) patients were previously healthy. Mean time from onset of illness to admission was 2.9 days; 61 (76%) patients were admitted early to the hospital (within 3 days after symptom onset). Respiratory distress, inspiratory retraction, and low percutaneous oxygen saturation (<93% while breathing room air) were significantly more frequent among patients with than without complications (p<0.01).
Infiltrates were more often localized (64 patients) than diffuse (16 patients). Unilateral localized infiltrates occurred more commonly in a lower lung field than in upper or middle fields, and unilateral infiltrates were more common in the right than left lung.
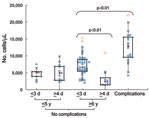
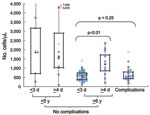
Clinical laboratory results are shown in Table 2. The neutrophil count was significantly higher in patients with complications than in others (Figure 1). Lymphopenia (<1,000 cells/μL) was characteristic in children with complications and in children who had no complications and were >6 years of age and admitted to the hospital on day 1–3 of illness (Figure 2). Lymphocyte count was significantly higher in the corresponding group with admission >4 days after onset. Serum immunoglobulin E concentration was high (>170 IU/mL) in both groups admitted on day 1–3, regardless of whether complications were present.
PCR detected bacteria in nasopharyngeal specimens from 41 (51%) patients. Organisms present included Streptococcus pneumoniae (25 patients), Haemophilus influenzae (28), and Mycoplasma pneumoniae and S. pyogenes (1 each); some patients had multiple organisms. In addition, rhinovirus was detected in 2 patients and enterovirus in 1.
Forty-nine (61%) patients required oxygen administration (mean duration 3.5 days) (Table 1). Oxygen supplementation was provided significantly more often to children who had than who did not have complications (15 [88%] vs. 34 [54%]; p<0.05). A total of 67 (84%) patients received oseltamivir, and 63 (79%) received antimicrobial drugs. Median time from onset of symptoms to initiation of oseltamivir treatment (4 mg/kg/d for 5 days) based on 20 applicable patients was 2 days, showing no differences between groups. Isoproterenol inhalation was needed only for patients with complications. In 1 patient who had an asthma attack, plastic bronchitis developed and the patient required invasive mechanical ventilation for 5 days.
All children recovered, with a median hospital stay of 6 days (Table 1). Hospitalization was longer for patients with than without complications (median 8 days vs. 6 days; p<0.01).
Our study has several limitations. Our PCR data from nasopharyngeal swabs cannot distinguish pathogens from colonizing organisms and cannot reliably guide decisions regarding antimicrobial drug treatment. Various reports have described invasive secondary bacterial infection with Staphylococcus aureus diagnosed from lower respiratory tract or blood specimens (7,8); such cultures were not obtained from all of the patients in our study. Moreover, pneumonia may have been underdiagnosed in our patients considering limited sensitivity of chest radiography compared with computed tomography (9).
Pediatricians should be aware that early diagnosis of influenza can enable prompt antiviral treatment of severe illness. All Japanese citizens have ready access to medical institutions through the national health insurance system. On November 13, 2009, the Japan Pediatric Society reported surveillance data concerning 60 pandemic (H1N1) 2009–associated deaths in children (10). Main causes of death were sudden death and rapidly progressive severe pneumonia. Testing practices, access, and policies regarding early administration of antiviral agents have protected many children from life-threatening pandemic (H1N1) 2009.
Dr Hasegawa is a fellow in the Department of General Pediatrics, Nerima-Hikarigaoka Hospital, Nihon University School of Medicine, in Tokyo. His primary research interests focus on general pediatric medicine; respiratory medicine, including asthma; and infectious diseases and clinical microbiology, particularly involving major respiratory tract pathogens.
Acknowledgment
This work was supported by a fourth fellowship from the Japanese Society for Pediatric Infectious Diseases (to M.H.) and by a grant from the Kawano Masanori Memorial Foundation for Promotion of Pediatrics (to T.T.).
References
- Hasegawa M, Hashimoto K, Morozumi M, Ubukata K, Takahashi T, Inamo Y. Spontaneous pneumomediastinum complicating pneumonia in children infected with the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. 2010;16:195–9. Epub 2009 Oct 14. DOIPubMedGoogle Scholar
- Hasegawa M, Inamo Y, Fuchigami T, Hashimoto K, Morozumi M, Ubukata K, Bronchial casts in 2009 pandemic influenza A (H1N1). Emerg Infect Dis. 2010;16:344–6.PubMedGoogle Scholar
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. DOIPubMedGoogle Scholar
- The Committee for the Guidelines in Management of Respiratory Infectious Diseases in Children. In: Uehara S, Sunakawa K, editors. Guidelines for the management of respiratory infectious diseases in children in Japan 2007. Tokyo: Japanese Society of Pediatric Pulmonology and Japanese Society for Pediatric Infectious Diseases; 2007. p. 56–7.
- Hamano-Hasegawa K, Morozumi M, Nakayama E, Chiba N, Murayama SY, Takayanagi R, Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–32. DOIPubMedGoogle Scholar
- Morozumi M, Nakayama E, Iwata S, Aoki Y, Hasegawa K, Kobayashi R, Simultaneous detection of pathogens in clinical samples from patients with community-acquired pneumonia by real-time PCR with pathogen-specific molecular beacon probes. J Clin Microbiol. 2006;44:1440–6. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection—United States, April–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:941–7.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Bacterial co-infections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4.PubMedGoogle Scholar
- Ou Q, Lu Y, Huang Q, Cheng X. Clinical analysis of 150 cases with the novel influenza A (H1N1) virus infection in Shanghai. Biosci Trends. 2009;3:127–30.PubMedGoogle Scholar
- Japan Pediatric Society. Emergency report of updated surveillance data regarding pandemic (H1N1) 2009 infection in Japanese children. 2009 [in Japanese] [cited 2009 Dec 12]. http://www.jpeds.or.jp/influenza/influenza_091113.pdf
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 2—February 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Takashi Takahashi, Laboratory of Infectious Diseases, Graduate School of Infection Control Sciences, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan
Top