Volume 19, Number 12—December 2013
Research
Spontaneous Generation of Infectious Prion Disease in Transgenic Mice
Figure 3
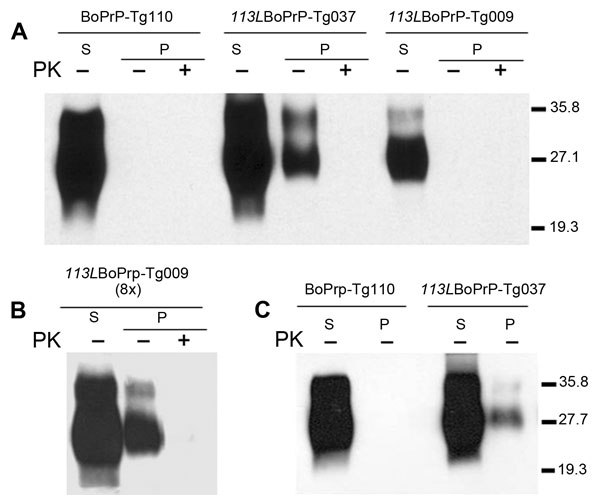
Figure 3. . . Host cellular prion protein (PrPC) solubility and proteinase K (PK) resistance studies in homozygous 113LBoPrP-Tg037, 113LBoPrP-Tg009, and control BoPrP-Tg110 mice. Western blot analysis with monoclonal antibody 2A11 of soluble (S) and insoluble (P) fractions obtained from mouse brain extracts (5% sarkosyl in phosphate-buffered saline, pH 7.4, previously cleared by centrifugation at 2,000 × g) after ultracentrifugation at 100,000 × g for 1 h. P fractions were treated with 5 μg/mL of PK (PK+) at 37°C for 60 min or left untreated (PK–). Panels A and B, show brain extracts from diseased 113LBoPrP-Tg037 mice or from 500-day-old 113LBoPrP-Tg009 and BoPrP-Tg110 mice. Panel C shows brain extracts from 30-day-old mice. In panels A and C, equivalent amounts of brain material were solubilized, centrifuged, and loaded onto the gel. In panel B, an 8-fold (8×) equivalent brain tissue mass was loaded to obtain equivalent PrPC signals for the other 2 mouse lines. 113L, leucine substitution at codon 113; BoPrP, bovine prion protein. Values on the right are molecular masses in kilodaltons.