Volume 19, Number 3—March 2013
Research
Effects of Vaccine Program against Pandemic Influenza A(H1N1) Virus, United States, 2009–2010
Figure 1
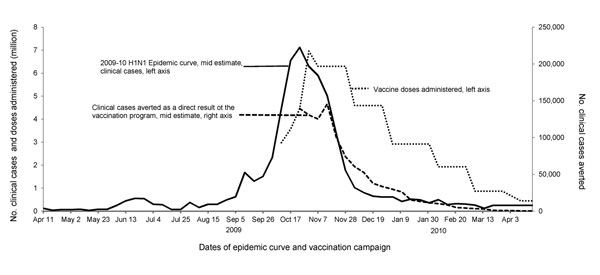
Figure 1. . . Weekly number of clinical cases of influenza A(H1N1)pdm09 virus infection, the number of vaccine doses administered, and the estimated number of cases averted over time because of the vaccination program. Midranges shown for epidemic curve and clinical cases; ranges provided in Table 3.
1Current affiliation: Merck & Co., Inc., Lansdale, Pennsylvania, USA.
Page created: February 12, 2013
Page updated: February 12, 2013
Page reviewed: February 12, 2013
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.