Volume 20, Number 6—June 2014
Dispatch
Infection with Mansonella perstans Nematodes in Buruli Ulcer Patients, Ghana
Abstract
During August 2010–December 2012, we conducted a study of patients in Ghana who had Buruli ulcer, caused by Mycobacterium ulcerans, and found that 23% were co-infected with Mansonella perstans nematodes; 13% of controls also had M. perstans infection. M. perstans co-infection should be considered in the diagnosis and treatment of Buruli ulcer.
Buruli ulcer, caused by Mycobacterium ulcerans, is a neglected tropical disease common in rural parts of West Africa. Infection with M. ulcerans causes disfiguring skin ulcers, mainly in children. The disease is highly focal, and in Ghana, cases are reported mainly from the humid and tropical southern regions, including Ashanti and Greater Accra (1). Recent studies suggest that aquatic invertebrates serve as a reservoir for M. ulcerans, although complete transmission pathways remain unknown (2,3). Aquatic insects infected with M. ulcerans can establish infection in mice by biting (4), but it is not clear that this is the cause of human infection (5). In southeastern Australia, evidence has been found linking infected mosquitoes with human cases (6,7), but proof of transmission is lacking.
Residents of regions in which Buruli ulcer is endemic are frequently exposed to parasitic infections such as filariasis. In Ghana, lymphatic filariasis caused by Wuchereria bancrofti nematodes is found in several regions to which Buruli ulcer is endemic, such as the Upper Denkyira District in the central region of Ghana, but its prevalence is unknown (8). The filarial nematode Mansonella perstans is endemic to countries in central and western Africa; its distribution overlaps that of other filarial nematodes W. bancrofti, Loa loa, and Onchocerca volvulus (9). Infective M. perstans larvae are transmitted through the bite of Culicoides midges (Diptera: Ceratopogonidae); the larvae develop over the course of months into adult worms that reside in serous cavities, particularly in the abdomen. M. perstans infection is not associated with a specific set of clinical signs and symptoms, but those attributed to this infection include acute swelling in the forearms, hands, and face that recedes in a few days and often recurs; itching with or without rash; arthralgia; and eosinophilia (9).
During an investigation into the immunopathogenesis of Buruli ulcer, we observed M. perstans nematodes in preparations of peripheral blood mononuclear cells from a patient. This finding led us to consider whether this organism was involved in the transmission or pathogenesis of M. ulcerans disease or if the finding was incidental. We then conducted a small case–control study to investigate the frequency of M. perstans co-infection in patients with M. ulcerans disease and the effect of this co-infection, if any, on patient response to antimicrobial drug therapy.
During August 2010–December 2012, we recruited all patients who had clinically suspected M. ulcerans infection and had attended a clinic in the Buruli ulcer–endemic Asante Akim North District in Ghana. Age- and sex-matched household contacts of patients were also asked to participate; all study participants were >5 years of age. The study protocol was approved by the ethics review committee of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology (CHRPE/91/10).
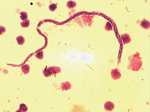
Whole blood samples were taken at baseline, at week 6, and at week 12 from 66 patients in whom the diagnosis of Buruli ulcer disease had been confirmed by PCR for the IS2404 repeat sequence specific for M. ulcerans (8); samples were also obtained from 20 household contacts at the same intervals. The samples were heparinized, and peripheral blood mononuclear cells were separated from 10-mL samples. Filarial infection was confirmed on a blood film stained with Giemsa and Delafield hematoxylin and examined for microfilariae at ×10 and ×40 magnification (the Knott technique; 10). M. perstans nematodes were distinguished from L. loa and W. bancrofti nematodes by their small size and the absence of a sheath (Figure 1).
Patients in whom M. ulcerans infection was found were treated with 10 mg/kg oral rifampin and 15 mg/kg intramuscular streptomycin, administered daily at village health posts under direct observation for 8 weeks (RS8 treatment). The patients were followed up every 2 weeks in the clinic and monitored for complete healing or recurrence of skin lesions. We compared the proportion of household controls versus the proportion of Buruli ulcer patients infected with M. perstans nematodes and the time to complete healing of M. ulcerans lesions in co-infected versus monoinfected patients. Categorical variables such as sex, clinical form of M. ulcerans lesion, and category of M. ulcerans lesion were compared by using the Fisher exact test, and cumulative healing was compared by using the log-rank test.
We found all forms of M. ulcerans disease among the group of patients; proportions of each type and category are shown in the Table. Of 66 patients with M. ulcerans disease, 15 (22.7%) were co-infected with M. perstans nematodes, whereas 4 (13%) of 30 household controls had M. perstans infection (p = 0.4 by Fisher exact test). Three patients in the co-infected group and none in the M. ulcerans–monoinfected group reported pruritus. No other clinical signs of M. perstans infection were found.
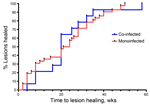
All 66 patients completed RS8 treatment, but 9 were lost to follow-up during the 12-month follow-up period. Buruli ulcer lesions healed completely in 14 co-infected patients by 58 weeks (median 20 weeks, 95% CI 14.6–30.2) and in 43 monoinfected patients by 50 weeks (median 21 weeks, 95% CI 16.7–25.5). We found no difference in cumulative time to healing for co-infected versus monoinfected patients (p>0.05 by log-rank test) (Figure 2). Buruli ulcer patients who had M. perstans nematodes co-infection were treated with doxycycline (200 mg) and ivermectin (150 μg/kg) daily for 6 weeks, starting during the second to fourth week of RS8 treatment. Viable microfilariae were still visible in peripheral blood mononuclear cell cultures from all co-infected patients after ivermectin and doxycycline treatment, but pruritus subsided in the 3 patients who had reported it.
We found co-infection with M. perstans in 23% of Buruli ulcer patients in a disease-endemic district in Ghana, but this prevalence was not significantly difference from prevalence among household contacts who served as controls (13%). As with Buruli ulcer, M. perstans filariasis is predominantly found in rural populations and infection begins in childhood; the highest infection rates are found in children 10–14 years of age (11), similar to those for children at highest risk for M. ulcerans infection. M. perstans infection occurs in Ghana and was seen in the Volta region of Ghana around Hohoe during the 1990s, but its prevalence is unknown (12), and no information is available about the average number of worms per infection. In Uganda, prevalence of M. perstans infection has been found to range from 0.4% to 50% (13).
M. perstans nematodes are transmitted by the bites of Culicoides midges, but it is not known whether M. perstans–infected midges can be co-infected with M. ulcerans. In a guinea pig model, skin penetration was shown to be a requirement for establishment of M. ulcerans disease (14), and it has been postulated that mosquito bites cause M. ulcerans disease in Australia (6). These organisms might share a common route of transmission, but our findings in this small study do not support this concept.
Our findings suggest that M. perstans nematodes are common in rural Ghana and coincidentally infect patients with M. ulcerans disease, necessitating the consideration of these organisms in the management plan of Buruli ulcer patients. Although often asymptomatic, M. perstans infection may cause eosinophilia, subcutaneous swellings, aches, pains, and skin rashes in a considerable proportion of patients (9). Because filarial nematodes are known to polarize the host immune responses from T-helper type 1 cells needed for protection against mycobacterial infections, toward humoral and T helper type-2 mediated immunity, we plan to undertake a study to investigate this interaction.
Dr Phillips is a senior lecturer at the Kwame Nkrumah University of Science and Technology. His research interest is the pathogenesis and management of M. ulcerans disease (Buruli ulcer).
Acknowledgments
We are grateful to the patients and contacts from the Asante Akim North District who agreed to be part of this study.
Funding for this work was provided by the European Community's Seventh Framework Programme under grant agreement no. 241500. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. R.O.P.’s research is funded under the United Kingdom Medical Research Council and the Department for International Development African Research Leader scheme reference MR/J01477X/1.
References
- Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006;367:1849–58 . DOIPubMedGoogle Scholar
- Williamson HR, Benbow ME, Campbell LP, Johnson CR, Sopoh G, Barogui Y, Detection of Mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2012;6:e1506.
- Williamson HR, Benbow ME, Nguyen KD, Beachboard DC, Kimbirauskas RK, McIntosh MD, Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl Trop Dis. 2008;2:e205. DOIPubMedGoogle Scholar
- Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, Legras P, Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol. 2002;68:4623–8. DOIPubMedGoogle Scholar
- Benbow ME, Williamson H, Kimbirauskas R, McIntosh MD, Kolar R, Quaye C, Aquatic invertebrates as unlikely vectors of Buruli ulcer disease. Emerg Infect Dis. 2008;14:1247–54 . DOIPubMedGoogle Scholar
- Johnson PD, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13:1653–60. DOIPubMedGoogle Scholar
- Lavender CJ, Fyfe JA, Azuolas J, Brown K, Evans RN, Ray LR, Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS Negl Trop Dis. 2011;5:e1305. DOIPubMedGoogle Scholar
- Hoerauf A, Specht S, Buttner M, Pfarr K, Mand S, Fimmers R, Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol (Berl). 2008;197:295–311. DOIPubMedGoogle Scholar
- Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl 1):S109–20. DOIPubMedGoogle Scholar
- Asio SM, Simonsen PE, Onapa AW. Mansonella perstans filariasis in Uganda: patterns of microfilaraemia and clinical manifestations in two endemic communities. Trans R Soc Trop Med Hyg. 2009;103:266–73. DOIPubMedGoogle Scholar
- Awadzi K, Hero M, Opoku O, Buttner DW, Gilles HM. The chemotherapy of onchocerciasis. XV. Studies with albendazole. Trop Med Parasitol. 1991;42:356–60 .PubMedGoogle Scholar
- Onapa AW, Simonsen PE, Baehr I, Pedersen EM. Rapid assessment of the geographical distribution of Mansonella perstans infections in Uganda, by screening schoolchildren for microfilariae. Ann Trop Med Parasitol. 2005;99:383–93. DOIPubMedGoogle Scholar
- Marsollier L, Aubry J, Milon G, Brodin P. Aquatic insects and transmission of Mycobacterium ulcerans. Med Sci (Paris). 2007;23:572–5. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleMedline reports that reference 8 "Hoerauf, Specht, Buttner, Pfarr, Mand, Fimmers, et al., 2008" was corrected in "Med Microbiol Immunol. 2008 Sep;197(3):335" (Note: Taylor, Mark J [added]).
Table of Contents – Volume 20, Number 6—June 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Richard O. Phillips, Kwame Nkrumah University of Science and Technology, School of Medical Sciences, Department of Medicine, Private Mail Bag, KNUST Kumasi, Ghana
Top