Volume 22, Number 10—October 2016
Letter
Cerebral Syphilitic Gumma within 5 Months of Syphilis in HIV-Infected Patient
To the Editor: Tertiary syphilis, including cerebral syphilitic gumma, usually occurs >10 years after contracting syphilis (1) and is a rare manifestation since the introduction of penicillin (2). However, progression of syphilis is reported to be faster in HIV-infected patients than in those without such infections (3). We report a case of cerebral syphilitic gumma in an HIV-1–infected patient for whom serum samples obtained as recently as 5 months earlier showed negative results for syphilis.
A 21-year-old man infected with HIV came to the AIDS Clinical Center, National Center for Global Health and Medicine (Tokyo, Japan), because of a 2-hour loss of consciousness. He reported an uncomfortable feeling at the back of his head and neck and eye fatigue that lasted for 1 week. His HIV-1 infection was well-controlled with an antiretroviral combination of tenofovir, emtricitabine, and dolutegravir. The patient had a CD4 count of 565 cells/μL and a viremia level below detectable limits (<20 copies/mL). He was not using any other medications.
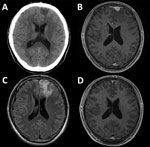
At examination, his vital signs were within reference ranges. Apart from a tongue bite, physical and neurologic examinations showed no abnormal findings. Results for chest radiograph, Holter electrocardiogram, and electroencephalogram were unremarkable. There were no abnormal ophthalmologic findings. Computed tomography of the brain showed a hypodense lesion at the left frontal lobe (Figure, panel A). Subsequent magnetic resonance imaging showed that the lesion (mass) was hypointense by gadolinium-enhanced, axial, T1-weighted imaging (Figure, panel B), hyperintense by T2-weighted imaging, and surrounded by extensive cerebral edema (Figure, panel C).
Symptomatic epilepsy caused by the mass was suspected to have caused the loss of consciousness. This conclusion was based on the intracranial mass, long duration of loss of consciousness, increase in creatine kinase level (471 U/L), and tongue bite.
When the HIV-1 infection was diagnosed in the patient 15 months earlier, results of serum rapid plasma reagin (RPR) and Treponema pallidum hemagglutination test (TPHA) were negative. However, during this examination, serum RPR and TPHA titers were 1:32 and 1:10,240, respectively. Results of cerebrospinal fluid (CSF) analysis were compatible with neurosyphilis (4,5) and showed a leukocyte count of 35 cells/μL (2 neutrophils/μL, 33 lymphocytes/μL), a total protein level of 30 mg/dL, a glucose level of 59 mg/dL (serum glucose level 92 mg/dL), an RPR titer of 1:<1, a TPHA titer of 1:160, and a fluorescent treponemal antibody-absorption titer of 1:32.
Cerebral syphilitic gumma was suspected on the basis of neurosyphilis and compatible imaging findings (6) and because other conditions, such as meningioma, primary central nervous system lymphoma, toxoplasmosis, cryptococcoma, tuberculoma, or brain abscess, were unlikely. These results were based on radiologic findings; high CD4 cell counts; and negative results for Epstein-Barr virus and malignant cells in CSF; serum cryptococcal antigen; CSF culture for bacteria, mycobacteria, and fungi; and interferon-γ release assay.
We did not perform a biopsy for the patient because of presumed high pretest probability of cerebral syphilitic gumma, the invasiveness of this complication, and the young age of the patient. However, successful therapy confirmed the diagnosis (3).
We empirically treated the patient with intravenous benzylpenicillin (24 million units/d for 14 consecutive days). Clinical symptoms improved shortly after treatment. At 2-month follow-up, all clinical symptoms and signs had resolved, and a 4-fold decrease in RPR titer was observed (RPR titer 1:8, TPHA titer 1:5,120) (7). The brain mass was substantially reduced, which confirmed the diagnosis of cerebral syphilitic gumma (Figure, panel D).
Gumma is a complication of long-term infection with T. pallidum, which develops 1–46 years after healing of secondary lesions; most cases develop within 15 years (1). However, for our patient, cerebral syphilitic gumma developed within 5 months after he contracted syphilis. After written informed consent was obtained, serum samples were obtained from the patient at his first and subsequent clinic visits and stored. Samples obtained at 11 months, 10 months, 9 months, 5 months, 11 weeks, and 5 weeks before detection of the brain mass were then tested retrospectively for RPR and TPHA titers. Results were negative at 11, 10, 9, and 5 months before detection of the brain mass, but TPHA titers became positive (1:80) at 11 weeks before presentation, and RPR titers became positive (1:16) at 5 weeks before presentation. Because RPR and TPHA titers can become positive as late as 6 weeks after infection (8), we believe that the patient contracted syphilis within 5 months before documentation of the cerebral mass.
In conclusion, we report an HIV-1–infected patient in whom cerebral syphilitic gumma developed within 5 months after contracting syphilis. Cerebral syphilitic gumma should be considered in the differential diagnosis of a cerebral lesion in sexually active patients even if they had recently contracted syphilis. Moreover, as guidelines recommend (9), screening of HIV-infected patients who are sexually active with multiple partners should be conducted every 3–6 months for early detection of syphilis and initiation of proper treatment to prevent transmission and progression to late syphilis.
Acknowledgments
We thank the clinical staff at the AIDS Clinical Center (Tokyo, Japan) for assistance.
This study was supported by Grant-in-Aids for AIDS research from the Japanese Ministry of Health, Labour, and Welfare (H23-AIDS-001 and H24-AIDS-003).
References
- Clark EG, Danbolt N. The Oslo study of the natural history of untreated syphilis; an epidemiologic investigation based on a restudy of the Boeck-Bruusgaard material; a review and appraisal. J Chronic Dis. 1955;2:311–44.DOIPubMedGoogle Scholar
- Noel CB, Moeketsi K, Kies B. Cavernous sinus syndrome, an atypical presentation of tertiary syphilis: case report and review of the literature. Clin Neurol Neurosurg. 2011;113:65–7.DOIPubMedGoogle Scholar
- Pialoux G, Vimont S, Moulignier A, Buteux M, Abraham B, Bonnard P. Effect of HIV infection on the course of syphilis. AIDS Rev. 2008;10:85–92.PubMedGoogle Scholar
- Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clin Infect Dis. 2009;48:816–21.DOIPubMedGoogle Scholar
- Castro R, Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: an evaluation of the Venereal Disease Research Laboratory (VDRL) and the Rapid Plasma Reagin (RPR) tests. J Clin Lab Anal. 2008;22:257–61.DOIPubMedGoogle Scholar
- Fargen KM, Alvernia JE, Lin CS, Melgar M. Cerebral syphilitic gummata: a case presentation and analysis of 156 reported cases. Neurosurgery. 2009;64:568–75, 575–6.DOIPubMedGoogle Scholar
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.PubMedGoogle Scholar
- Janier M, Hegyi V, Dupin N, Unemo M, Tiplica GS, Potočnik M, et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014;28:1581–93.DOIPubMedGoogle Scholar
- Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207, quiz CE1–4.PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 10—October 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Takeshi Nishijima, AIDS Clinical Center, National Center for Global Health and Medicine, 1-21-1, Toyama, Shinjuku, Tokyo 162-8655, Japan
Top