Volume 22, Number 2—February 2016
Dispatch
Effectiveness of Meningococcal B Vaccine against Endemic Hypervirulent Neisseria meningitidis W Strain, England
Abstract
Serum samples from children immunized with a meningococcal serogroup B vaccine demonstrated potent serum bactericidal antibody activity against the hypervirulent Neisseria meningitidis serogroup W strain circulating in England. The recent introduction of this vaccine into the United Kingdom national immunization program should also help protect infants against this endemic strain.
Invasive meningococcal disease (IMD) has been declining in the United Kingdom since the early 2000s (1). Historically, serogroup W Neisseria meningitides (MenW) have been causal organisms for 1%–2% of IMD cases annually. An increase in invasive MenW disease associated with travel to the Hajj pilgrimage route during 2000–2002 was rapidly controlled after the introduction of mandatory vaccination for pilgrims (2). Since 2009, however, laboratory-confirmed MenW cases in England have increased each year across all age groups after rapid spread of a single endemic hypervirulent sequence type (ST) 11 clonal complex (MenW:cc11) strain (3). This strain has caused severe illness with unusual clinical manifestations and, for the first time in more than a decade, was associated with fatal outcomes among infants and young children.
Since the Hajj outbreak, several countries in Latin America, Africa, and the Far East have reported an increase in MenW disease and ongoing endemic transmission (3). In Chile, MenW has replaced serogroup B (MenB) as the most prevalent cause of IMD, identified in 58% of cases in 2012 (4). In Europe, an increase in MenW disease has not been observed in other countries although, in 2012, a cluster of cases related to MenW:cc11 in France was associated with travel to sub-Saharan Africa (5).
In England, the meningococcal quadrivalent conjugate vaccine (MenACWY, covering serogroups A, C, W, and Y) has historically been recommended for high-risk persons and travelers to disease-endemic regions and for controlling outbreaks (6). Beginning on September 1, 2015, a novel, protein-based, multicomponent vaccine, Bexsero (GSK Vaccines, Siena, Italy), has been offered as part of the routine immunization program in the United Kingdom; the vaccine is given to infants in 3 doses at 2, 4, and 12 months of age (https://www.gov.uk/government/publications/menb-vaccination-introduction-from-1-september-2015). Bexsero is composed of NHBA (neisserial heparin binding antigen), NadA (Neisseria adhesin A) and fHbp (factor H binding protein), with meningococcal outer membrane vesicles from MenB strain from an outbreak in New Zealand (7). Immunization with Bexsero induces bactericidal antibodies against all vaccine antigens (8). Although this vaccine has been licensed for prevention of MenB disease (the most prevalent capsular group causing IMD in Europe), alleles for some or all of the vaccine antigens are also found among non-MenB meningococci, independently of the capsule. Therefore, antibodies raised by these antigens could induce complement-mediated killing of other meningococcal groups, including the endemic MenW cc11 strain.
Recently, we reported the predominance of non–cross-protective PorA (P1.5,2) and fHbp variants (variant 2 peptide 22) among endemic MenW:cc11 isolates (3). The other primary Bexsero antigens (NadA and NHBA), however, could potentially afford protection. We therefore assessed 1) the NadA and NHBA genotypic status of endemic MenW:cc11 isolates, and 2) the serum bactericidal antibody (SBA) activity against clinical MenW:cc11 isolates using serum samples from infants immunized with Bexsero.
A total of 73 invasive MenW:cc11 isolates from England and Wales were received by the Public Health England Meningococcal Reference Unit during July 2010–June 2013. These isolates were queried within the Meningitis Research Foundation Meningococcus Genome Library (http://pubmlst.org/perl/bigsdb/bigsdb.pl?db = pubmlst_neisseria_mrfgenomes) for nadA and nhba and, where present, their respective allelic and peptide variants.
We used an SBA using human complement (hSBA) against 6 invasive MenW:cc11 isolates from patients 4 months–91 years of age in whom meningitis or septicemia was diagnosed in different regions of England and Wales during 2011–2012. SBA titers were expressed as the reciprocal of the serum dilution corresponding to >50% bacterial killing. We used pooled serum samples from phase 2 clinical trials involving infants immunized with Bexsero at 2, 3, and 4 months or 2, 4, and 6 months and after administration of a booster at 12, 18, or 24 months (8). Pooled prevaccination serum samples from 180 infants were used as negative controls, and pooled serum samples from 10 randomly selected adolescents who received a single MenACWY conjugate vaccine (GSK Vaccines) dose as positive controls.
Of the 6 isolates tested, 4 possessed nadA allele 5 (for peptide NadA-2/3.6) and nhba allele 17 (for NHBA peptide 29). Of the remaining 2 isolates, 1 isolate had a nadA allele (allele 146) that differed at a single nucleotide (C476A), causing a single amino acid change (T159K; peptide NadA-2/3.130); the other isolate had an nhba allele (allele 72) that differed at a single nucleotide (A376C), causing a single amino acid change (T126P; peptide 96).
hSBA titers were high and were >1:32 against all 6 MenW isolates, independently of the immunization schedule (Table). After the booster, higher hSBA titers were obtained than those from primary immunization, and similar responses were comparable to those among adolescents who had received a single MenACWY dose. Preimmunization serum samples showed no detectable hSBA titers against any of the 6 isolates (Table).
The ability of the antibodies raised by Bexsero antigens to induce SBA activity against any given meningococcal isolate depends on the presence, level of surface expression, and sequence diversity of the respective antigens. Bexsero strain coverage can be predicted by using the Meningococcal Antigen Typing System, an ELISA which measures the level of antigen expression and antigenic diversity compared with the antigen in the vaccine (9). However, the correlation between the relative potency estimated by this typing system and the ability of a meningococcal isolate to be killed by serum from immunized persons has only been defined for MenB strains.
We found that MenW:cc11 isolates causing invasive disease in England and Wales possessed alleles for NadA-2/3 peptide variants that are predicted to be highly cross-protective with the Bexsero NadA variant (10). The isolates also possessed alleles for NHBA peptide 29 which, although different from peptide 2 in Bexsero, has the potential to induce cross-protection even if NHBA-containing cross-protective epitopes have not been defined yet. Antibodies against Bexsero antigens can act synergistically and, therefore, the complement-mediated bactericidal killing observed could be mediated by antibodies against NadA, NHBA, or both.
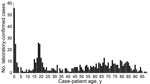
In England, the ongoing MenW increase is similar to the MenC:cc11 outbreak in the mid-1990s, which was eventually controlled through mass vaccination (11). The increase in MenW cases has led to the rapid introduction of a national adolescent MenACWY conjugate vaccination program in August 2015 (https://www.gov.uk/government/news/new-meningococcal-vaccination-programme-expected-to-save-lives). In adolescents, a single MenACWY dose would provide direct protection and, by targeting the age group most likely to carry meningococci (12), could also provide indirect protection against MenW (Figure) and the other 3 capsular groups by reducing carriage and onward transmission to others.
Conversely, infants would require >2 MenACWY doses starting at 2 months of age, because MenW cases increase from birth and peak at 7 months of age before declining. Bexsero, which is predicted to protect against 73%–88% of invasive MenB isolates in England and Wales (9,13), could offer additional protection against MenB, which causes more cases among infants and toddlers than the other meningococcal serogroups. Among infants (<1 year of age), 101 MenB cases were reported during 2014–2015, compared with 21 MenW, 4 MenY, and 1 MenC; among toddlers, (1–4 years of age), 139 MenB cases were reported, compared with 18 MenW, 5 MenY, and 0 MenC. The difference in adolescents (15–19 years of age) is less pronounced, but 36 MenB cases were reported in this age group during 2014–2015, compared with 25 MenW, 14 MenY and 3 MenC cases (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/476989/hpr38-3915.pdf). However, the effectiveness of Bexsero against meningococcal carriage and, therefore, its ability to provide herd protection, which is a major objective of an adolescent programme, is less certain than with conjugate vaccines (14). These observations and our results support the recent implementation of both the adolescent MenACWY conjugate and infant MenB immunization programmes in the UK.
Dr. Ladhani is a pediatric infectious disease consultant and a clinical epidemiologist at Public Health England, London, United Kingdom. He is the clinical lead for enhanced national surveillance of several vaccine-preventable infections in England and Wales and has a particular research interest in the direct and indirect population impact of conjugate and other vaccines.
Acknowledgment
J.F. and R.B. perform contract research on behalf of Public Health England for GSK, Novartis, Pfizer, Sanofi Pasteur and Sanofi Pasteur MSD, and S.N.L. performs contract research for the same companies on behalf of St. George’s University of London. None of the authors have received any personal remuneration. M.M.G., A.B., and M.P. are employees of GSK Vaccines, which manufactures the MenB vaccine, Bexsero.
References
- Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30:3710–6 . DOIGoogle Scholar
- Hahné SJ, Gray SJ, Aguilera JF, Crowcroft NS, Nichols T, Kaczmarski EB, W135 meningococcal disease in England and Wales associated with Hajj 2000 and 2001. Lancet. 2002;359:582–3 . DOIGoogle Scholar
- Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2014;•••:578–85.
- Araya P, Fernandez J, Del CF, Seoane M, Ibarz-Pavon AB, Barra G, Neisseria meningitidis ST-11 clonal complex, Chile 2012. Emerg Infect Dis. 2015;21:339–41 . DOIGoogle Scholar
- Parent du Châtelet I, Barboza P, Taha MK. W135 invasive meningococcal infections imported from Sub-Saharan Africa to France, January to April 2012. Euro Surveill. 2012;•••:17.
- Department of Health. Pneumococcal. In: Salisbury D, Ramsay M, Noakes K, editors. Immunisation against infectious disease. Norwick (UK): The Stationery Office; 2006 [updated 2011]. p. 295–313
- Isaacs D, McVernon J. Introducing a new group B meningococcus vaccine. BMJ. 2014;348:g2415 . DOIPubMedGoogle Scholar
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307:573–82 . DOIPubMedGoogle Scholar
- Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13:416–25 . DOIPubMedGoogle Scholar
- Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–54 . DOIPubMedGoogle Scholar
- Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine. 2009;27(Suppl 2):B20–9 . DOIPubMedGoogle Scholar
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61 . DOIPubMedGoogle Scholar
- Frosi G, Biolchi A, Sapio ML, Rigat F, Gilchrist S, Lucidarme J, Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31:4968–74 . DOIPubMedGoogle Scholar
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 22, Number 2—February 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Shamez Ladhani, Immunisation Department, Public Health England, 61 Colindale Ave, London NW9 5EQ, UK
Top