Volume 23, Number 2—February 2017
Research Letter
Diffuse Unilateral Subacute Neuroretinitis Caused by Ancylostoma Hookworm
Abstract
Diffuse unilateral subacute neuroretinitis is an ocular infectious disease caused by several distinct nematodes. Definite identification of the involved nematodes is rarely achieved. We report on the molecular-based genetic identification of an Ancylostoma ceylanicum hookworm implicated in a case of diffuse unilateral subacute neuroretinitis in a child.
Diffuse unilateral subacute neuroretinitis (DUSN) is an ocular infectious disease caused by migrating larvae of nematodes. Patients typically have vitritis, papillitis, and gray-white retinal lesions (1,2). DUSN primarily occurs in the United States, the Caribbean, and South America, although several cases also have been reported in Europe, Africa, India, and China (2). Several nematodes can induce DUSN, in particular Ancylostoma spp., Baylisascaris procyonis, and Toxocara canis (3), but the actual cause remains unknown. Because the nematodes are only rarely surgically extracted from the eye, a definite identification is hardly ever achieved (3,4). Noninvasive laser therapy is the treatment of choice for DUSN because it leads to the death of the nematode, thereby stopping the inflammatory process (1). Anthelminthic therapy with albendazole also has been described as successful, albeit primarily in cases where a worm cannot be visualized in the patient’s eye (5). Left untreated, DUSN can progress toward optic nerve atrophy and permanent vision loss.
In their larval form, hookworms infect their hosts by penetrating intact skin. The larvae circulate through the blood to the heart and then reach the lungs, before being coughed up and swallowed, thus entering the gastrointestinal tract. In the intestines, the larvae develop into adult worms and start to reproduce. This leads to the fecal shedding of eggs into the environment. A. ceylanicum is a zoonotic hookworm predominantly found in dogs and cats in Southeast Asia, India, and Australia (6). It is the only animal hookworm species known to cause patent intestinal infections in humans (6).
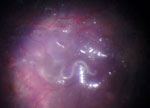
We report on a 10-year-old boy born in Columbia who had been living with his foster parents in Germany for the previous 6 years. He had acute loss of vision in his right eye. Ophthalmoscopy revealed retinal vasculitis, exudative retinal detachment, and proliferative vitreoretinopathy. Because of the retinal detachment, we performed a vitrectomy, during which a white worm of ≈10 mm in length was observed moving in the subretinal space (Figure). During surgical removal, the worm was completely destroyed.
We isolated DNA from the intraoperative rinsing fluid and applied previously described PCR assays to target the mitochondrial 12S rRNA, Cox1, and intergenic transcribed spacer 1 and 2 of various nematodes (7–9). We then sequenced the PCR products obtained. Phylogenetic analysis with the intergenic transcribed spacer 1 and 2 sequences (GenBank accession no. KM066110.1) identified the worm as A. ceylanicum (Technical Appendix). A blastn search (https://blast.ncbi.nlm.nih.gov) with the Cox1 sequence (GenBank accession no. KM066109.1) showed 99% and 87% identity with A. ceylanicum and A. caninum, respectively. The 12Sr RNA sequence (GenBank accession no. KM066111.1) for A. ceylanicum revealed 94% and 92% identity to A. caninum and A. duodenale, respectively.
Additional diagnostic results included negative serum antibody tests for the parasites Fasciola hepatica, Strongyloides spp., Trichinella spp., and Taenia solium; negative blood samples for filariasis; and 3 negative stool samples for intestinal helminths. An ELISA result for serum antibodies to the helminth Toxocara canis (DRG, Marburg, Germany) was weakly positive, whereas the confirmatory immunoblot test result (Lobio Diagnostics, Lyon, France) was negative.
In addition to performing a vitrectomy, retinotomy, and implantation of silicon oil, we started the patient on antiinflammatory therapy with oral prednisone. Referring to the treatment recommendation of a case series (5) and ensuring that no additional worm could survive, we administered a 30-day course of anthelminthic therapy with albendazole, even though no signs of additional organ manifestation or blood eosinophilia were observed. Unfortunately, a permanent loss of visual acuity to 0.05 could not be averted.
DUSN is an inflammatory eye disease caused by migrating nematode larvae. Because of the surgical intervention necessary in the case we describe, we were able to amplify and determine DNA sequences of the hookworm A. ceylanicum. The finding of a hookworm in DUSN seems plausible because Ancylostoma spp. nematodes have been repeatedly proposed as an etiologic agent in DUSN (1,3,4).
The source and time of infection in our patient remains unclear. After the patient’s adoption and his move from Columbia to Germany at 4 years of age, the patient and his adoptive family spent vacations in Spain but never traveled outside Europe. We do not know whether the infection was acquired in Columbia or Spain and subsequently survived (e.g., in a hypobiotic state) or whether the infection was acquired in Germany. Although the definitive source and time of infection cannot be confirmed, molecular methods nevertheless unquestionably identified the species A. ceylanicum.
In this case, we obtained a positive ELISA result for Toxocara canis, another helminth implicated in DUSN (3). Because the confirmatory immunoblot test was negative, we assume the ELISA result to most likely have been caused by unspecific cross-reactions. In the past, tests based on serologic testing alone might have falsely attributed nematodes to DUSN. In future cases of exudative retinal detachment caused by DUSN, intraoperative material should be used for molecular studies to identify the responsible nematode.
Dr. Poppert is a medical microbiologist and is currently working as attending physician at the Department of Infectious Diseases at the University Medical Center, Hamburg-Eppendorf, Germany. His main field of research is the development and application of molecular tests for the detection of human pathogens.
References
- Garcia CA, Sabrosa NA, Gomes AB, Segundo PS, Garcia Filho CA, Sabrosa AS. Diffuse unilateral subacute neuroretinitis—DUSN. Int Ophthalmol Clin. 2008;48:119–29.
- de Amorim Garcia Filho CA, Gomes AH, de A Garcia Soares AC, de Amorim Garcia CA. Clinical features of 121 patients with diffuse unilateral subacute neuroretinitis. Am J Ophthalmol. 2012;153:743–9. DOIPubMedGoogle Scholar
- Arevalo JF, Garcia RA, Tata LS, de Amorim Garcia CA, Orefice F, Curi ALL, et al. Diffuse unilateral subacute neuroretinitis (DUSN). In: Arevalo JF, editor. Retinal and choroidal manifestation of selected systemic diseases. New York: Springer Science + Business Media; 2013. p. 23–35.
- Otranto D, Eberhard ML. Zoonotic helminths affecting the human eye. Parasit Vectors. 2011;4:41. DOIPubMedGoogle Scholar
- Souza EC, Casella AM, Nakashima Y, Monteiro ML. Clinical features and outcomes of patients with diffuse unilateral subacute neuroretinitis treated with oral albendazole. Am J Ophthalmol. 2005;140:437–45. DOIPubMedGoogle Scholar
- Carroll SM, Grove DI. Experimental infection of humans with Ancylostoma ceylanicum: clinical, parasitological, haematological and immunological findings. Trop Geogr Med. 1986;38:38–45.PubMedGoogle Scholar
- Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. DOIPubMedGoogle Scholar
- Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54:165–73. DOIPubMedGoogle Scholar
- Gasser RB, Stevenson LA, Chilton NB, Nansen P, Bucknell DG, Beveridge I. Species markers for equine strongyles detected in intergenic rDNA by PCR-RFLP. Mol Cell Probes. 1996;10:371–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 23, Number 2—February 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Markus Hufnagel, Center for Pediatrics and Adolescent Medicine, Freiburg University Medical Center, Mathildenstr. 1, Freiburg 79106, Germany
Top