Volume 3, Number 2—June 1997
Synopsis
Hantaviruses: A Global Disease Problem
Abstract
Hantaviruses are carried by numerous rodent species throughout the world. In 1993, a previously unknown group of hantaviruses emerged in the United States as the cause of an acute respiratory disease now termed hantavirus pulmonary syndrome (HPS). Before then, hantaviruses were known as the etiologic agents of hemorrhagic fever with renal syndrome, a disease that occurs almost entirely in the Eastern Hemisphere. Since the discovery of the HPS-causing hantaviruses, intense investigation of the ecology and epidemiology of hantaviruses has led to the discovery of many other novel hantaviruses. Their ubiquity and potential for causing severe human illness make these viruses an important public health concern; we reviewed the distribution, ecology, disease potential, and genetic spectrum.
The genus Hantavirus, family Bunyaviridae, comprises at least 14 viruses, including those that cause hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (Table 1). Several tentative members of the genus are known, and others will surely emerge as their natural ecology is further explored. Hantaviruses are primarily rodent-borne, although other animal species harboring hantaviruses have been reported. Unlike all other viruses in the family, hantaviruses are not transmitted by arthropod vectors but (most frequently) from inhalation of virus-contaminated aerosols of rodent excreta (1). Human-to-human transmission of hantaviruses has not been documented, except as noted below.
The recognition of a previously unknown group of hantaviruses as the cause of HPS in 1993 is an example of virus emergence due to environmental factors favoring of the natural reservoir; a larger reservoir increases opportunities for human infection. We reviewed the global distribution of hantaviruses, their potential to cause disease, and their relationships to each other and to their rodent hosts.
"Hemorrhagic fever with renal syndrome" denotes a group of clinically similar illnesses that occur throughout the Eurasian landmass and adjoining areas (2,3). HFRS includes diseases previously known as Korean hemorrhagic fever, epidemic hemorrhagic fever, and nephropathia epidemica (4). Although these diseases were recognized in Asia perhaps for centuries, HFRS first came to the attention of western physicians when approximately 3,200 cases occurred from 1951 to 1954 among United Nations forces in Korea (2,5). Other outbreaks of what is believed to have been HFRS were reported in Russia in 1913 and 1932, among Japanese troops in Manchuria in 1932 (2,6), and in Sweden in 1934 (7,8). In the early 1940s, a viral etiology for HFRS was suggested by Russian and Japanese investigators who injected persons with filtered urine or serum from patients with naturally acquired disease (2). These studies also provided the first clues to the natural reservoir of hantaviruses: the Japanese investigators claimed to produce disease in humans by injecting bacteria-free filtrates of tissues from Apodemus agrarius or mites that fed on the Apodemus mice. Mite transmission was never conclusively demonstrated by other investigators, and it was not until 1978 that a rodent reservoir for HFRS-causing viruses was confirmed by investigators who demonstrated that patient sera reacted with antigen in lung sections of wild-caught Apodemus agrarius and that the virus could be passed from rodent to rodent (9). The successful propagation of Hantaan (HTN) virus in cell culture in 1981 provided the first opportunity to study this pathogen systematically (10). The history of HFRS has been explored (2,11,12).
HPS was first described in 1993 when a cluster of cases of adult fatal respiratory distress of unknown origin occurred in the Four Corners region of the United States (New Mexico, Arizona, Colorado, and Utah). The unexpected finding that sera from patients reacted with hantaviral antigens was quickly followed by the genetic identification of a novel hantavirus in patients' tissues and in rodents trapped near patients' homes (13-15).
Approximately 150,000 to 200,000 cases of HFRS involving hospitalization are reported each year throughout the world, with more than half in China (16). Russia and Korea also report hundreds to thousands of HFRS cases each year. Most remaining cases (hundreds per year) are found in Japan, Finland, Sweden, Bulgaria, Greece, Hungary, France, and the Balkan countries formerly constituting Yugoslavia (16). Depending in part on which hantavirus is responsible for the illness, HFRS can appear as a mild, moderate, or severe disease (Table 2). Death rates range from less than 0.1% for HFRS caused by Puumala (PUU) virus to approximately 5% to 10% for HFRS caused by HTN virus (16). The clinical course of severe HFRS involves five overlapping stages: febrile, hypotensive, oliguric, diuretic, and convalescent; it is not uncommon, however, for one or more of these stages to be inapparent or absent. The onset of the disease is usually sudden with intense headache, backache, fever, and chills. Hemorrhage, if it occurs, is manifested during the febrile phase as a flushing of the face or injection of the conjunctiva and mucous membranes. A petechial rash may also appear, commonly on the palate and axillary skin folds. Sudden and extreme albuminuria, around day 4, is characteristic of severe HFRS. As the febrile stage ends, hypotension can abruptly develop and last for hours or days, during which nausea and vomiting are common. One-third of deaths occur during this phase because of vascular leakage and acute shock. Almost half of all deaths occur during the subsequent (oliguric) phase because of hypervolemia. Patients who survive and progress to the diuretic phase show improved renal function but may still die of shock or pulmonary complications. The final (convalescent) phase can last weeks to months before recovery is complete (3,5,12).
More than 250 cases of HPS have been reported throughout North and South America. Although the disease has many features (e.g., a febrile prodrome, thrombocytopenia, and leukocytosis) in common with HFRS (Table 2), in HPS capillary leakage is localized exclusively in the lungs, rather than in the retroperitoneal space, and the kidneys are largely unaffected. Most of the 174 cases of HPS in the United States and Canada have been caused by Sin Nombre (SN) virus. In HPS, death occurs from shock and cardiac complications, even with adequate tissue oxygenation. Cases of HPS in the southeastern United States, as well as many in South America, are caused by a newly recognized clade (a group that shares a common ancestor) of viruses that includes Bayou (BAY), Black Creek Canal (BCC), and Andes viruses. As with HFRS, clinical differences can be observed among patients with HPS caused by different hantaviruses. For example, although HPS due to SN virus infection can sometimes be associated with renal insufficiency after prolonged hypoperfusion, renal impairment is only rarely observed early in disease, and chemical evidence of skeletal muscle inflammation (increased serum levels of the muscle enzyme creatine kinase) is rare (17). In contrast, both renal insufficiency and elevated creatine kinase levels are observed at much higher frequency, although not universally, with Andes, BAY, and BCC virus infections (18-20; J. Davis, J. Cortes, and C. Barclay, pers. comm.). In an outbreak of HPS recently described in Paraguay, a novel hantavirus, carried by Calomys laucha, was identified as the etiologic agent (21). The relationship of this virus to other HPS-causing hantaviruses remains to be established.
Hantavirus infection is apparently not deleterious to its rodent reservoir host and is associated with a brisk antibody response against the virion envelope and core proteins and chronic, probably lifelong infection. In natural populations, most infections occur through age-dependent horizontal route(s). The highest antibody prevalence is observed in large (mature) animals. A striking male predilection for hantavirus infection is observed in some rodent species such as harvest mice and deer mice, but not in urban rats (Rattus norvegicus) (22-24). Horizontal transmission among cage-mates was experimentally demonstrated (25), but vertical transmission from dam to pup is negligible or absent both in wild and experimental settings (22,24,25).
Outbreaks of hantaviral disease have been associated with changes in rodent population densities, which may vary greatly across time, both seasonally and from year to year. Cycles respond to such extrinsic factors as interspecific competition, climatic changes, and predation. Spring and summer outbreaks of HFRS in agricultural settings in Asia and Europe are linked to human contact with field rodents through the planting and harvesting of crops (16,26). PUU outbreaks in Scandinavia and the HPS outbreak in the Four Corners region of the United States were associated with natural rodent population increases, followed by invasion of buildings by rodents (27,28). The ecologic events that led to 1994 and 1996 outbreaks of Andes virus-HPS in Patagonia, a region in southern South America, are being investigated. Human interventions, such as the introduction of Old World plant species (e.g., rosas mosquetas and Scottish brougham) to Patagonia, with associated alteration in rodent population dynamics, have been suggested as possible factors. Recent fires and a mild winter in Argentina's Rio Negro and Chubut Provinces may also have had a positive effect on the carrier rodent, the colilargo, Oligoryzomys longicaudatus (M. Christie and O. Pearson, pers. comm.).
Although the aerosol route of infection is undoubtedly the most common means of transmission among rodents and to humans, virus transmission by bite may occur among certain rodents (29) and may also occasionally result in human infection (30) (often inside a closed space, such as a rodent-infested grain silo, garage, or outbuilding used for food storage). Epidemiologic investigations have linked virus exposure to such activities as heavy farm work, threshing, sleeping on the ground, and military exercises. Indoor exposure was linked to invasion of homes by field rodents during cold weather or to nesting of rodents in or near dwellings (16,31). Genetic sequencing of rodent- and patient-associated viruses has been used to pinpoint the precise locations of human infections, which has supported the role of indoor exposure in hantavirus transmission (32,33). Many hantavirus infections have occurred in persons of lower socioeconomic status because poorer housing conditions and agricultural activities favor closer contact between humans and rodents. However, suburbanization, wilderness camping, and other outdoor recreational activities have spread infection to persons of middle and upper incomes.
Nosocomial transmission of hantaviruses has not been documented until very recently (34) and must be regarded as rare. However, viruses have been isolated from blood and urine of HFRS patients, so exposure to bodily fluids of infected persons could result in secondary transmission. Only rarely have multiple North American HPS cases been associated with particular households or buildings. During recent outbreaks of HPS in South America, however, clustering of cases in households and among personal contacts appeared to be more common (M. Christie, pers. comm.). During a recent outbreak of Andes-virus-associated HPS in Patagonia, a Buenos Aires physician apparently contracted the infection after minimal exposure to infected patient blood (34; D.A. Pirola, pers. comm.). An adolescent patient in Buenos Aires apparently contracted hantavirus infection from her parents, who were infected in Patagonia. This unprecedented observation of apparent person-to-person spread of a hantavirus clearly requires laboratory confirmation, especially by careful comparative analysis of the viral sequences (32,33).
Hantaviruses have also caused several laboratory-associated outbreaks of HFRS. Laboratory-acquired infections were traced to persistently infected rats obtained from breeders (35-37), to wild-caught, naturally infected rodents (38-40), or to experimentally infected rodents (39). No illnesses due to laboratory infections have been reported among workers using cell-culture adapted viruses, although asymptomatic seroconversions have been documented (40).
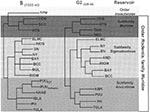
The worldwide distribution of rodents known to harbor hantaviruses (Table 1) suggests great disease-causing potential. Each hantavirus appears to have a single predominant natural reservoir. With rare exception, the phylogenetic interrelationships among the viruses and those of their predominant host show remarkable concordance (Figure; 41). These observations suggest that hantaviruses do not adapt readily to new hosts and that they are closely adapted for success in their host, possibly because of thousands of years of coexistence. As many as three hantaviruses can be found in a particular geographic site, each circulating in its own rodent reservoir, with no apparent evolutionary influence on one another (42).
All known hantaviruses, except Thotta-palayam (TPM) virus, have been isolated or detected in murid rodents. Because only one isolate of TPM virus was made from a shrew (Order Insectivora), it is not clear if Suncus is the true primary reservoir or an example of a "spillover" host, i.e., a secondary host infected through contact with the primary host. Spillover is common in sympatric murid rodents, including those identified as the predominant carrier of another hantavirus; thus, the opportunity for genetic exchange among hantaviruses is present in nature. Spillover hosts are believed to have little or no impact on hantaviral distribution or associated disease. However, rodents other than the primary reservoirs can play an important carrier role. For example, Microtus rossiaemeri-dionalis may play a role in maintenance of Tula virus in some settings (43), and Peromyscus leucopus and Peromyscus boylii can be important reservoirs for SN virus in the western United States (T. Yates and B. Hjelle, unpub. data). Apparent spillover may also be the result of laboratory errors such as polymerase chain reaction (PCR) contamination or misidentification of rodent species. However, spillover is probably under-appreciated in many studies that rely on reverse transcriptase PCR for identifying specific viruses because many primer pairs may not detect an unexpected spillover virus. In either case, because mistaken identities and cell culture contaminations with other hantaviruses have occasionally been reported, investigators should verify unusual findings to prevent further confusion.
Hantaviruses have been characterized by a combination of antigenic and genetic methods. For viruses propagated in cell culture, the plaque-reduction neutralization test is the most sensitive serologic assay for differentiation (44,45); nine hantaviruses have been defined by this test: HTN, Seoul (SEO), PUU, Prospect Hill, Dobrava-Belgrade (DOB), Thailand, TPM, SN, and BCC viruses (44-48). Genetic relationships among hantaviruses are mirrored in their antigenic properties. A direct correlation between genetic and antigenic relationships is difficult; however, it can be assumed that the plaque-reduction neutralization test measures differences in the M segment gene products, i.e., the G1 and G2 envelope glycoproteins. Comparing the deduced G1 and G2 amino acid sequences, therefore, may provide clues to the antigenic as well as genetic diversity among hantaviruses.
Of characterized hantavirus isolates, SEO virus is the most genetically homogeneous. Isolates of SEO virus, regardless of their geographic origin, display M segment nucleotide and deduced amino acid sequence homologies of approximately 95%, and 99%, respectively (41,47). A reported exception, the R22 isolate from China, had a slightly lower homology; however, the original data suggest that an error in the nucleotide sequence, resulting in a frame shift reading error, may account for almost all of the additional changes. PUU virus isolates vary the most, with M segment nucleotide and amino acid sequence homologies of 83% and 94% observed between a Finnish and Russian isolate. HTN virus also appears to be quite stable in nature. Comparing the M segment sequences of prototype HTN virus (from Apodemus) and those of two human isolates obtained at a 6-year interval from HFRS patients in Korea produced nucleotide and deduced amino acid sequence homologies of 94% and 97%, respectively (48). For SN virus, comparing the complete M or S segment sequences of three strains from California or New Mexico produced homologies of 87% to 99%. Partial nucleotide sequence comparisons of the M or S segments of SN viruses from adjacent counties in California, detected in deer mice captured 19 years apart, were 97.5% homologous (49). Similarly, a retrospective analysis of archived tissue samples collected in Mono County, California, in 1983 showed viruses with partial M and S segment nucleotide sequence homologies of about 87% with SN from an 1993 HPS patient from New Mexico (50). In all cases, the amino acid sequences encoded by these genes differed between cognate proteins by much less than 5%. These values are similar to those observed among strains of HTN virus. Studies have just begun to appear in which the nature of quasispecies in natural rodent hosts is defined (43,51). Such investigations should provide more definitive data concerning the genetic diversity among hantaviruses in nature.
Phylogenetic trees derived by comparing complete or partial S (Figure), M, or L segment nucleotide sequences (41,52,53) show two major lineages of hantaviruses, one leading to HTN, SEO, Thailand, and DOB viruses, and one leading to PUU, Prospect Hill, SN, and other New World hantaviruses. TPM virus, the first hantavirus isolated in cell culture (54), may be the most antigenically and genetically disparate member of the genus; however, comparison of the complete nucleotide sequence of the TPM S segment (A. Toney, B. Meyer, C. Schmaljohn, unpub. data) suggests that TPM virus is more closely related to HTN, SEO, and DOB viruses than to any of the other viruses in the genus (Figure). Nucleotide sequence homologies of the M and S segments of any two hantaviruses have approximately the same degree of divergence between each of the three segments, which suggests similar evolutionary rates for these two gene segments. A slightly higher homology among L segments sequenced to date perhaps indicates a greater need for conservation of either RNA or protein functions (47). Point mutations appear to account for most of the genetic drift among hantaviruses. Recombination has not been reported for hantaviruses, although segment reassortment within a particular species appears common (52,55). The exchange of gene segments is suggested to be nonrandom, with a higher propensity for M segment swapping, than for S or L (55). Whether it contributes to the pathogenesis of hantaviruses is not known, but reassortment certainly provides an avenue for more rapid accumulation of changes than could occur by point mutation. There is no evidence that reassortment can occur between different species of hantaviruses; however, this has not been studied systematically.
Murid rodents have probably harbored inapparent hantavirus infections for thousands, perhaps millions of years. It is likely that the genus Hantavirus evolved in the Old World and that viruses were carried by rodents across the Bering land bridge when they migrated during the Oligocene, and into South America in the Pliocene (71). Humans are incidental hosts, the victims of spillover infections from the natural host rodents. One of the two major forms of hantaviral diseases is endemic in each hemisphere. Both HFRS and HPS can be divided into distinct clinical subtypes, and the viral strain is a key determinant of the severity and nature of the clinical abnormalities. Not covered in this review are clinical studies of HFRS and HPS patients, which suggest that pathogenesis may be immunologic and may be mediated by cytokine responses (72). New outbreaks with novel hantavirus strains are still being uncovered, especially in South America. However, the largest clinical caseload and largest number of deaths occur in Asia and Europe.
Dr. Schmaljohn is chief, Department of Molecular Virology, USAMRID. Current research interests include the development of molecular vaccines for hantaviruses, filoviruses, and flaviviruses.
Dr. Hjelle has been active in studies of the molecular biology, evolution, epidemiology, and clinical aspects of hantavirus disease. His laboratory is a reference diagnostic center for hantavirus infections of humans and animals and has recently received funding to develop innovative vaccine strategies against HPS and other emerging viral diseases.
References
- Lee H, van der Groen G. Hemorrhagic fever with renal syndrome. Prog Med Virol. 1989;36:62–102.PubMedGoogle Scholar
- World Health Organization. Haemorrhagic fever with renal syndrome: memorandum from a WHO meeting. Bull World Health Organ. 1983;61:269–75.PubMedGoogle Scholar
- Gajdusek DC, Goldfarb LG, Goldgaber D. Bibliography of hemorrhagic fever with renal syndrome. Second Edition. Bethesda (MD): National Institutes of Health; 1987 Pub No. 88-3603.
- Casals J, Henderson BE, Hoogstraakm G. J Infect Dis. 1969;122:437–53.A review of Soviet viral hemorrhagic fevers
- Zetterholm SG. Akuta nefriter simulerande akuta bukfall. Svenska Lakartidningen 1934;31.
- Myhrman G. En njursjukdom med egenartad symptombild. Nord Med Tidskr. 1934;7:793–4.
- Lee H, Lee P, Johnson K. J Infect Dis. 1978;137:298–308.Isolation of the etiologic agent of Korean hemorrhagic feverPubMedGoogle Scholar
- French G, Foulke R, Brand O, Eddy G. Korean hemorrhagic fever: propagation of the etiologic agent in a cell line of human origin. Science. 1981;211:1046–8. DOIPubMedGoogle Scholar
- McKee K, LeDuc J, Peters C. Hantaviruses. In: Belshe RB, Belshe RB, editors. Textbook of human virology. St Louis: Mosby Year Book 1991:615-32.
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Science. 1993;262:914–7.Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness DOIPubMedGoogle Scholar
- Ksiazek TG, Peters CJ, Rollin PE, Zaki S, Nichol S, Spiropoulou C, Am J Trop Med Hyg. 1995;52:117–23.Identification of a new North American hantavirus that causes acute pulmonary insufficiencyPubMedGoogle Scholar
- Schmaljohn AL, Li D, Negley DL, Bressler DS, Turell MJ, Korch GW, Virology. 1995;206:963–72.Isolation and initial characterization of a newfound hantavirus from California DOIPubMedGoogle Scholar
- Lee HW. Epidemiology and pathogenesis of hemorrhagic fever with renal syndrome. In: Elliott RM, editor. The Bunyaviridae. New York: Plenum Press, 1996:253-67.
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, N Engl J Med. 1994;330:949–55.Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease DOIPubMedGoogle Scholar
- Khan AS, Spiropoulou CF, Morzunov S, Zaki SR, Kohn MA, Nawas SR, J Med Virol. 1995;46:281–6.Fatal illness associated with a new hantavirus in Louisiana DOIPubMedGoogle Scholar
- Khan AS, Gaviria JM, Rollin PE, Hlady WG, Ksiazek TG, Armstrong LR, Am J Med. 1996;100:46–8.Hantavirus pulmonary syndrome in Florida: association with the newly identified Black Creek Canal virus DOIPubMedGoogle Scholar
- Hjelle B, Goade D, Torrez-Martínez N, Lang-Williams M, Kim J, Harris RL, Clin Infect Dis. 1996;23:495–500.Hantavirus pulmonary syndrome, renal insufficiency and myositis associated with infection by Bayou hantavirusPubMedGoogle Scholar
- Williams R, Bryan R, Mills H, Palma I, Vera I, Velasquez F, Hantavirus pulmonary syndrome in western Paraguay. [suppl]. Am J Trop Med Hyg. 1996;:55.
- Mills J, Ksiazek T, Ellis B, Rollin P, Nichol S, Yates T, Patterns of association with host and habitat: antibody reactivity with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am J Trop Med Hyg. In press.
- Hjelle B, Anderson B, Torrez-Martínez N, Song W, Gannon WF. L. YT. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology. 1995;207:452–9. DOIPubMedGoogle Scholar
- Childs J, Korch G, Glass G, LeDuc J, Shah K. Am J Epidemiol. 1987;126:55–68.Epizootiology of hantavirus infections in Baltimore: isolation of a virus from Norway rats and characteristics of infected rat populationsPubMedGoogle Scholar
- Lee H, Lee P, Baek L, Song C, Seong I. Intraspecific transmission of Hantaan virus, etiologic agent of Korean hemorrhagic fever, in the rodent Apodemus agrarius. Am J Trop Med Hyg. 1981;30:1106–12.PubMedGoogle Scholar
- Chen H-X, Qiu F-X, Dong B-J, Ji S-Z, Li Y-T, Wang Y, Epidemiologic studies on hemorrhagic fever with renal syndrome in China. J Infect Dis. 1986;154:394–8.PubMedGoogle Scholar
- Niklasson B, LeDuc J. Epidemiology of nephropathia epidemica in Sweden. J Infect Dis. 1987;155:269–76.PubMedGoogle Scholar
- Parmenter R, Vigil R. The hantavirus epidemic in the southwest: an assessment of autumn rodent densities and population demographics in central and northern New Mexico. Atlanta (GA): 1993 Report to the Federal Centers for Disease Control and Prevention.
- Glass G, Childs J, Korch G, LeDuc J. Epidemiol Infect. 1988;101:459–72.Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus) DOIPubMedGoogle Scholar
- Dournon E, Moriniere B, Matheron S, Girard P, Gonzalez J, Hirsch F, HFRS after a wild rodent bite in the hautesavoie--and risk of exposure to Hantaan-like virus in Paris laboratory. Lancet. 1984;1:676–7. DOIGoogle Scholar
- Armstrong L, Zaki S, Goldoft M, Todd R, Khan A, Khabbaz R, J Infect Dis. 1995;172:1166.Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structuresPubMedGoogle Scholar
- Hjelle B, Torrez-Martínez N, Koster FT, Jay M, Ascher MS, Brown T,
- Jay M, Hjelle B, Davis R, Ascher M, Baylies HN, Reilly K, Clin Infect Dis. 1996;22:841–4.Occupational exposure leading to hantavirus pulmonary syndrome in a utility company employeePubMedGoogle Scholar
- Enria D, Padula P, Segura EL, Pini N, Edelstein A, Riva Posse C, Hantavirus pulmonary syndrome in Argentina: possibility of person to person transmission. Medicina (B Aires). 1996;56:709–11.PubMedGoogle Scholar
- Umenai T, Lee P, Toyoda T, Yoshinaga K, Lee H, Saito T, Lancet. 1979;1:1314–6.Korean hemorrhagic fever in staff in an animal laboratory DOIPubMedGoogle Scholar
- Desmyter J, LeDuc J, Johnson K, Brasseur F, Deckers C, Van Ypersele de Strihou C. Lancet. 1983;2:1445–8.Laboratory rat associated outbreak of haemorrhagic fever with renal syndrome due to Hantaan-like virus in Belgium DOIPubMedGoogle Scholar
- Lloyd G, Bowen E, Jones N. Lancet. 1984; (
May ):1775–6.HFRS outbreak associated with laboratory rats in UK - Brummer-Korvenkontio M, Vaheri A, von Bonsdorff C-H, Vuorimies J, Manni T, Penttinen K, J Infect Dis. 1980;141:131–4.Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infectionPubMedGoogle Scholar
- Lee H, Johnson K. J Infect Dis. 1982;146:645–51.Laboratory-acquired infections with hantaan virus, the etiologic agent of Korean hemorrhagic feverPubMedGoogle Scholar
- Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1994;43(RR-7):1–2.Laboratory management of agents associated with hantavirus pulmonary syndrome: interim biosafety guidelines
- Xiao SY, Leduc JW, Chu YK, Schmaljohn CS. Virology. 1994;198:205–17.Phylo-genetic analyses of virus isolates in the genus Hantavirus, family Bunyaviridae DOIPubMedGoogle Scholar
- Rawlings J, Torrez-Martínez N, Neill S, Moore G, Hicks B, Pichuantes S, Am J Trop Med Hyg. 1996;55:672–9.Cocirculation of multiple hantaviruses in Texas, with characterization of the S genome of a previously-undescribed virus of cotton rats (Sigmodon hispidus)PubMedGoogle Scholar
- Plyusnin A, Vapalahti O, Lankinen H, Lehvaslaiho H, Apekina N, Myasnikov Y, J Virol. 1994;68:7833–9.Tula virus: a newly detected hantavirus carried by European common volesPubMedGoogle Scholar
- Schmaljohn C, Hasty S, Dalrymple J, LeDuc J, Lee H, von Bonsdorff C-H, Science. 1985;227:1041–4.Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome DOIPubMedGoogle Scholar
- Chu Y-K, Rossi C, LeDuc J, Lee H, Schmaljohn C, Dalrymple J. Virology. 1994;198:196–204.Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae DOIPubMedGoogle Scholar
- Chu Y-K, Jennings G, Schmaljohn A, Elgh F, Hjelle B, Lee HW, J Infect Dis. 1995;172:1581–4.Cross-neutralization of hantaviruses with immune sera from experimentally-infected animals and from hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome patientsPubMedGoogle Scholar
- Schmaljohn CS. Molecular Biology of Hantaviruses. In: Elliott RM, editor. The Bunyaviridae. New York: Plenum Press, 1996:63-90.
- Schmaljohn C, Arikawa J, Hasty S, Rasmussen L, Lee WH, Lee PW, J Gen Virol. 1988;69:1949–55.Conservation of antigenic properties and sequences encoding the envelope proteins of prototype hantaan virus and two virus isolates from Korean haemorrhagic fever patients DOIPubMedGoogle Scholar
- Jay M, Ascher MS, Chomel BB, Madon M, Sesline D, Enge B, Sero-epidemiological studies of hantavirus infection among wild rodents in California. Emerg Infect Dis. 1997;▪▪▪:3.
- Nerurkar VR, Song JW, Song KJ, Nagle JW, Hjelle B, Jenison S, Virology. 1994;204:563–8.Genetic evidence for a hantavirus enzootic in deer mice (Peromyscus maniculatus) captured a decade before the recognition of hantavirus pulmonary syndrome DOIPubMedGoogle Scholar
- Rowe JE, St Jeor SC, Riolo J, Otteson EW, Monroe MC, Henderson WW, Virology. 1995;213:122–30.Coexistence of several novel hantaviruses in rodents indigenous to North America DOIPubMedGoogle Scholar
- Li D, Schmaljohn A, Anderson K, Schmaljohn C. Virology. 1995;206:973–83.Complete nucleotide sequences of the M and S segments of two hantavirus isolates from California: evidence for reassortment in nature among viruses related to hantavirus pulmonary syndrome DOIPubMedGoogle Scholar
- Spiropoulou CF, Morzunov S, Feldmann H, Sanchez A, Peters CJ, Nichol ST. Virology. 1994;200:715–23.Genome structure and variability of a virus causing hantavirus pulmonary syndrome DOIPubMedGoogle Scholar
- Carey D, Reuben R, Panicker K, Shope R, Myers R. Indian J Med Res. 1971;59:1758–60.Thottapalayam virus: a presumptive arbovirus isolated from a shrew in IndiaPubMedGoogle Scholar
- Henderson WW, Monroe MC, St Jeor SC, Thayer WP, Rowe JE, Peters CJ, Virology. 1995;214:602–10.Naturally occurring Sin Nombre virus genetic reassortants DOIPubMedGoogle Scholar
- Hjelle B, Jenison S, Torrez-Martínez N, Yamada T, Nolte K, Zumwalt R, J Virol. 1994;68:592–6.A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relation-ships to known hantavirusesPubMedGoogle Scholar
- Hjelle B, Jenison S, Goade D, Green W, Feddersen R, Scott A. Crit Rev Clin Lab Sci. 1995;32:469–508.Hantaviruses: clinical, microbiologic, and epidemiologic aspects DOIPubMedGoogle Scholar
- Avsic-Zupanc T, Xiao SY, Stojanovic R, Gligic A, van der Groen G, LeDuc JW. J Med Virol. 1992;38:132–7.Characterization of Dobrava virus: a Hantavirus from Slovenia, Yugoslavia DOIPubMedGoogle Scholar
- Lee H, Baek L, Johnson K. J Infect Dis. 1982;146:638–44.Isolation of hantaan virus, the etiologic agent of Korean hemorrhagic fever from wild urban ratsPubMedGoogle Scholar
- Elwell M, Ward G, Tingpalapong M, LeDuc J. Southeast Asian J Trop Med Public Health. 1985;16:349–54.Serologic evidence of hantaan-like virus in rodents and man in ThailandPubMedGoogle Scholar
- Lee P, Amyx H, Yanagihara R, Gajdusek D, Goldgaber D, Gibbs C Jr. J Infect Dis. 1985;152:826–9.Partial characterization of Prospect Hill virus isolated from meadow voles in the United StatesPubMedGoogle Scholar
- Hörling J, Chizhikov V, Lundkvist Å, Jonsson M, Ivanov L, Dekonenko A, J Gen Virol. 1996;77:687–94.Khabarovsk virus: a phylogenetically and serologically distinct hantavirus isolated from Microtus fortis trapped in far-east Russia DOIPubMedGoogle Scholar
- Elliott LH, Ksiazek TG, Rollin PE, Spiropoulou CF, Morzunov S, Monroe M, Am J Trop Med Hyg. 1994;51:102–8.Isolation of the causative agent of hantavirus pulmonary syndromePubMedGoogle Scholar
- Song J-W, Baek L-J, Gajdusek DC, Yanagihara R, Gavrilovskaya I, Luft BJ, Lancet. 1994;344:1637.Isolation of pathogenic hantavirus from white footed mouse (Peromyscus leucopus) DOIPubMedGoogle Scholar
- Ravkov EV, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. Virology. 1995;210:482–9.Genetic and serologic analysis of Black Creek Canal virus and its association with human disease and Sigmodon hispidus infection DOIPubMedGoogle Scholar
- Torrez-Martínez N, Song W, Hjelle B. Virology. 1995;211:336–8.Nucleotide sequence analysis of the M genomic segment of El Moro Canyon hantavirus: antigenic distinction from Four Corners hantavirus DOIPubMedGoogle Scholar
- Morzunov SP, Feldmann H, Spiropoulou CF, Semenova VA, Rollin PE, Ksiazek TG, J Virol. 1995;69:1980–3.A newly recognized virus associated with a fatal case of hantavirus pulmonary syndrome in LouisianaPubMedGoogle Scholar
- Plyusnin A, Vapalahti O, Lundkvist A, Henttonen H, Vaheri A. Lancet. 1996;347:1835.Newly recognised hantavirus in Siberian lemmings [letter] DOIPubMedGoogle Scholar
- López N, Padula P, Rossi C, Lázaro ME, Franze-Fernández MT. Virology. 1996;220:223–6.Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina DOIPubMedGoogle Scholar
- Song W, Torrez-Martínez N, Irwin W, Harrison J, Davis R, Ascher M, J Gen Virol. 1995;76:3195–9.Isla Vista virus: a genetically novel hantavirus of the California vole Microtus californicus DOIPubMedGoogle Scholar
- Hjelle B, Torrez-Martínez N, Koster FT. Lancet. 1996;347:57.Hantavirus pulmonary syndrome-related virus from Bolivia DOIPubMedGoogle Scholar
- Mertz G, Hjelle B, Bryan R. Hantavirus infection. In: Fauci A, Schrier R, editors. Advances in internal medicine. Chicago: Mosby Year Book Inc., 1996:373-425.
Figure
Tables
Cite This ArticleTable of Contents – Volume 3, Number 2—June 1997
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Connie Schmaljohn, Virology Division, USAMRID, Fort Detrick, Frederick, MD 21702-5011; fax: 301-619-2439
Top