Volume 5, Number 1—February 1999
Synopsis
Using Monoclonal Antibodies to Prevent Mucosal Transmission of Epidemic Infectious Diseases
Figure 2
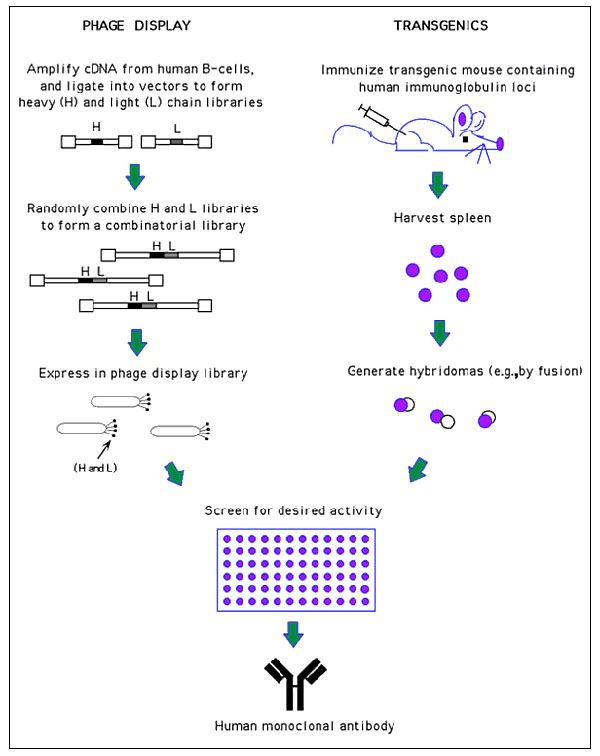
Figure 2. Generation of human monoclonal antibodies. (Phage display) Heavy and light chain cDNA isolated from human B-cells is used to generate a combinatorial library in which random heavy (H) and light chain (L) pairings are expressed on the surface of phage. These phage can then be screened for antigen binding by traditional techniques (e.g., ELISA). Since only the antigen binding region is used in the phage display process, the selected clone is then placed into an appropriate expression vector to produce a full antibody molecule. (Transgenics) Genetically manipulated mice have been produced with inactivated endogenous immuno- globulin genes, and with unrearranged human immunoglobulin gene segments introduced (90,91). These mice are then immunized with antigen, and hybridomas are produced by traditional routes. (See refs. 88, 89 for more technical information on these two methods and refs. 92, 93 for comparisons of these two methods).
References
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. DOIPubMedGoogle Scholar
- Casadevall A, Scharff M. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–61.PubMedGoogle Scholar
- Casadevall A. Antibody-based therapies for emerging infectious diseases. Emerg Infect Dis. 1996;2:200–8. DOIPubMedGoogle Scholar
- Cross A. Intravenous immunoglobulins to prevent and treat infectious diseases. In: Atassi MZ, GS Bixler GSJ, editors. Immunobiology of proteins and peptides VIII. New York: Plenum Press; 1995.
- Lederberg J, Shope R, Oaks S. Emerging Infections. Washington: National Academy Press; 1992.
- Mims C, Dimmock N, Nash A, Stephen J. Mims' pathogenesis of infectious disease. 4th ed. San Diego: Academic Press; 1995.
- Mo H, Stamatatos L, Ip JE, Barbas CF, Parren PWHI, Burton DR, Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–74.PubMedGoogle Scholar
- Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402.PubMedGoogle Scholar
- Anderson D, Yunis E. "Trojan horse" leukocytes in AIDS. N Engl J Med. 1983;309:984–5.
- Ogra P. Mucosal immunoprophylaxis: an introductory overview. In: Kiyono H, Ogra P, McGhee J, editors. Mucosal vaccines. New York: Academic Press; 1996. p. 1-14.
- Cone RA, Whaley KJ. Monoclonal antibodies for reproductive health: Part I. Preventing sexual transmission of disease and pregnancy with topically applied antibodies. Am J Reprod Immunol. 1994;32:114–31.PubMedGoogle Scholar
- Saltzman WM. Antibodies for treating and preventing disease: the potential role of polymeric controlled release. Crit Rev Ther Drug Carrier Syst. 1993;10:111–42.PubMedGoogle Scholar
- Silverstein A. History of immunology. In: Paul W, editor. Fundamental immunology. 2nd ed. New York: Raven Press Ltd.; 1989.
- Dimmock N. Neutralization of animal viruses. Curr Top Microbiol Immunol. 1993;183:1–149.PubMedGoogle Scholar
- Igarashi A, Fukuoka T, Fukai K. Passive immunization of mice with rabbit antisera against Chikungunya virus and its components. Biken J. 1971;14:353–5.PubMedGoogle Scholar
- CytoGam package insert. Gaithersburg (MD): MedImmune Inc.
- Men RH, Bray M, Lai CJ. Carboxy-terminally truncated dengue virus envelope glycoproteins expressed on the cell surface and secreted extracellularly exhibit increased immunogenicity in mice. J Virol. 1991;65:1400–7.PubMedGoogle Scholar
- Mikhailov VV, Borisevich IV, Chernikova NK, Potryvaeva NV, Krasnianskii VP. The evaluation in hamadryas baboons of the possibility for the specific prevention of Ebola fever. Vopr Virusol. 1994;39:82–4.PubMedGoogle Scholar
- Arikawa J, Yao JS, Yoshimatsu K, Takashima I, Hashimoto N. Protective role of antigenic sites on the envelope protein of Hantaan virus defined by monoclonal antibodies. Arch Virol. 1992;126:271–81. DOIPubMedGoogle Scholar
- Eis-Hübinger A, Schmidt D, Schneweis K. Anti-glycoprotein B monoclonal antibody protects mice against genital herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J Gen Virol. 1993;74:379–85. DOIPubMedGoogle Scholar
- Atherton SS. Protection from retinal necrosis by passive transfer of monoclonal antibody specific for herpes simplex virus glycoprotein D. Curr Eye Res. 1992;11:45–52. DOIPubMedGoogle Scholar
- Safrit JT, Fung MS, Andrews CA, Braun DG, Sun WN, Chang TW, hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS. 1993;7:15–21. DOIPubMedGoogle Scholar
- Stapleton JT. Passive immunization against hepatitis A. Vaccine. 1992;10:S45–7. DOIPubMedGoogle Scholar
- McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC, Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996;61:1358–64. DOIPubMedGoogle Scholar
- Okuno Y, Matsumoto K, Isegawa Y, Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol. 1994;68:517–20.PubMedGoogle Scholar
- Jahrling PB, Peters CJ. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun. 1984;44:528–33.PubMedGoogle Scholar
- Giraudon P, Wild T. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144:46–58. DOIPubMedGoogle Scholar
- Chanock R, Crowe J, Murphy B, Burron D. Human monoclonal antibody Fab fragments cloned from combinatorial libraries: potential usefulness in prevention and/or treatment of major human viral diseases. Infect Agents Dis. 1993;2:118–31.PubMedGoogle Scholar
- Dietzschold B, Kao M, Zheng YM, Chen ZY, Maul G, Fu ZF, Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system [published erratum appears in Proc Natl Acad Sci U S A 1992;89:9365]. Proc Natl Acad Sci U S A 1992;89:7252-6. 630.
- Sherry B, Li XY, Tyler KL, Cullen JM, Virgin HW IV. Lymphocytes protect against and are not required for reovirus-induced myocarditis. J Virol. 1993;67:6119–24.PubMedGoogle Scholar
- Niklasson BS, Meadors GF, Peters CJ. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. APMIS. 1984;92:197–200.
- MedImmune reports fourth set of clinical results evaluating MEDI-493 [press release]. Gaithersburg (MD): MedImmune; May 6, 1997.
- The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–9. DOIPubMedGoogle Scholar
- Neumann-Haefelin D, Neumann-Haefelin C, Petersen EE, Luthardt T, Hass R. Passive immunization against rubella: studies on the effectiveness of rubella-immuno-globulin after intranasal infection with rubella vaccination virus. Dtsch Med Wochenschr. 1975;100:177–81. DOIPubMedGoogle Scholar
- Brunell P, Ross A, Miller L. B K. Prevention of varicella by zoster immune globulin. N Engl J Med. 1969;280:1191–4.PubMedGoogle Scholar
- Danes L, Hruskova J. Efficiency testing of passive immunization against Venezuelan equine encephalomyelitis in mice. Acta Virol. 1969;13:554–6.PubMedGoogle Scholar
- Johnson RC, Kodner C, Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986;53:713–4.PubMedGoogle Scholar
- Sato Y, Sato H. Further characterization of Japanese acellular pertussis vaccine prepared in 1988 by 6 Japanese manufacturers. Tokai J Exp Clin Med. 1988;13:79–88.PubMedGoogle Scholar
- Kaukoranta-Tolvanen SE, Laurila AL, Saikku P, Leinonen M, Laitinen K. Experimental Chlamydia pneumoniae infection in mice: effect of reinfection and passive immunization. Microb Pathog. 1995;18:279–88. DOIPubMedGoogle Scholar
- Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–14.PubMedGoogle Scholar
- Raff HV, Bradley C, Brady W, Donaldson K, Lipsich L, Maloney G, Comparison of functional activities between IgG1 and IgM class-switched human monoclonal antibodies reactive with group B streptococci or Escherichia coli K1. J Infect Dis. 1991;163:346–54.PubMedGoogle Scholar
- Drabick J, Narayanan R, Williams J, LeDuc J, Nacy C. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–7. DOIPubMedGoogle Scholar
- Shigeoka AO, Pincus SH, Rote NS, Hill HR. Protective efficacy of hybridoma type-specific antibody against experimental infection with group-B Streptococcus. J Infect Dis. 1984;149:363–72.PubMedGoogle Scholar
- Schreiber JR, Barrus V, Cates KL, Siber GR. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type B. J Infect Dis. 1986;153:8–16.PubMedGoogle Scholar
- Hayatsu E, Kawakubo Y, Yayoshi M, Araake M, Wakai M, Yoshida A, Immunological responses of hamsters in the acquired immune state to Mycoplasma pneumoniae infection. Microbiol Immunol. 1981;25:1255–63.PubMedGoogle Scholar
- Brodeur BR, Larose Y, Tsang P, Hamel J, Ashton F, Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985;50:510–6.PubMedGoogle Scholar
- Harmon RC, Rutherford RL, Wu HM, Collins MS. Monoclonal antibody-mediated protection and neutralization of motility in experimental Proteus mirabilis infection. Infect Immun. 1989;57:1936–41.PubMedGoogle Scholar
- Fisher MW. A polyvalent human gamma-globulin immune to Pseudomonas aeruginosa: passive protection of mice against lethal infection. J Infect Dis. 1977;136:S181–5.PubMedGoogle Scholar
- Svenson SB, Nurminen M, Lindberg AA. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979;25:863–72.PubMedGoogle Scholar
- Adamus G, Mulczyk M, Witkowska D, Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980;30:321–4.PubMedGoogle Scholar
- Scott DF, Best GK, Kling JM, Thompson MR, Adinolfi LE, Bonventre PF. Passive protection of rabbits infected with toxic shock syndrome-associated strains of Staphylococcus aureus by monoclonal antibody to toxic shock syndrome toxin 1. Reviews of Infectious Diseases 1989;11:S214-7; discussion S217-8.
- Swendsen CL, Johnson W. Humoral immunity to Streptococcus pneumoniae induced by a pneumococcal ribosomal protein fraction. Infect Immun. 1976;14:345–54.PubMedGoogle Scholar
- Azadegan AA, Schell RF, LeFrock JL. Immune serum confers protection against syphilitic infection on hamsters. Infect Immun. 1983;42:42–7.PubMedGoogle Scholar
- Motin V, Nakajima R, Smirnov G, Brubaker R. Passive immunity to Yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun. 1994;62:4192–201.PubMedGoogle Scholar
- Friedlander AM, Welkos SL, Worsham PL, Andrews GP, Heath DG, Anderson GW Jr, Relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin Infect Dis. 1995;21:S178–81.PubMedGoogle Scholar
- Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M. Immunoprotection against systemic candidiasis in mice. Int Immunol. 1995;7:785–96. DOIPubMedGoogle Scholar
- Nussbaum G, Yuan R, Casadevall A, Scharff MD. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J Exp Med. 1996;183:1905–9. DOIPubMedGoogle Scholar
- Diggs CL, Hines F, Wellde BT. Plasmodium falciparum: passive immunization of Aotus lemurinus griseimembra with immune serum. Exp Parasitol. 1995;80:291–6. DOIPubMedGoogle Scholar
- Johnson AM, McDonald PJ, Neoh SH. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J Protozool. 1983;30:351–6.PubMedGoogle Scholar
- Garrett L. The coming plague. New York: Penguin Books; 1995.
- Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–79.PubMedGoogle Scholar
- Cone R. Mucus. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Mucosal immunology. 2nd ed. New York: Academic Press; 1999.
- Lamm M. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–40. DOIPubMedGoogle Scholar
- Whaley KJ, Zeitlin L, Barratt RA, Hoen TE, Cone RA. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–9.PubMedGoogle Scholar
- Zeitlin L, Whaley KJ, Sanna PP, Moench TR, Bastidas R, De Logu A, Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab')2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225:213–5. DOIPubMedGoogle Scholar
- Zeitlin L. Topical methods for preventing genital herpes infection in the mouse. Reproductive biology. [dissertation]. Baltimore: The Johns Hopkins University; 1996.
- Jakeman K, Smith H, Sweet C. Mechanism of immunity to influenza: maternal and passive neonatal protection following immunization of adult ferrets with a live vaccinia-influenza virus hemagglutinin recombinant but not with recombinants containing other influenza virus proteins. J Gen Virol. 1989;70:1523–31. DOIPubMedGoogle Scholar
- Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–44. DOIPubMedGoogle Scholar
- Davidson GP, Whyte PB, Daniels E, Franklin K, Nunan H, McCloud PI, Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus [see comments]. Lancet. 1989;2:709–12. DOIPubMedGoogle Scholar
- Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217–23.PubMedGoogle Scholar
- Weltzin R, Traina-Dorge V, Soike K, Zhang JY, Mack P, Soman G, Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis. 1996;174:256–61.PubMedGoogle Scholar
- Peterson EM, Cheng X, Motin VL, de la Maza LM. Effect of immunoglobulin G isotype on the infectivity of Chlamydia trachomatis in a mouse model of intravaginal infection. Infect Immun. 1997;65:2693–9.PubMedGoogle Scholar
- Lyerly DM, Bostwick EF, Binion SB, Wilkins TD. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–8.PubMedGoogle Scholar
- Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988;318:1240–3.PubMedGoogle Scholar
- Booth V, Ashley F, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–7.PubMedGoogle Scholar
- Tacket C, Binion S, Bostwick E, Losonsky G, Roy M, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–83.PubMedGoogle Scholar
- Ramisse F, Szatanik M, Binder P, Alonso J-M. Passive local immunotherapy of experimental staphylococcal pneumonia with human intravenous immunoglobulin. J Infect Dis. 1993;168:1030–3.PubMedGoogle Scholar
- Ma JK, Hunjan M, Smith R, Kelly C, Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect Immun. 1990;58:3407–14.PubMedGoogle Scholar
- Apter FM, Michetti P, Winner LSD, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–85.PubMedGoogle Scholar
- Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–24.PubMedGoogle Scholar
- Perryman LE, Riggs MW, Mason PH, Fayer R. Kinetics of Crytosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–9.PubMedGoogle Scholar
- Ma JK, Hein MB. Immunotherapeutic potential of antibodies produced in plants. Trends Biotechnol. 1995;13:522–7. DOIPubMedGoogle Scholar
- Chintalacharuvu KR, Morrison SL. Production of secretory immunoglobulin A by a single mammalian cell. Proc Natl Acad Sci U S A. 1997;94:6364–8. DOIPubMedGoogle Scholar
- Kilian M, Russel M. Function of mucosal immunoglobulins. In: Ogra P, Mestecky J, Lamm M, Strober W, McGhee J, Bienenstock J, editors. Handbook of mucosal immunology. San Diego: Academic Press; 1994. p. 127-37.
- Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–79.PubMedGoogle Scholar
- Winter G, Griffiths A, Hawkins R, Hoogenboom H. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–55. DOIPubMedGoogle Scholar
- Burton D, Barbas C. Human antibodies from combinatorial libraries. Adv Immunol. 1994;57:191–280. DOIPubMedGoogle Scholar
- Williamson R, Burioni R, Sanna P, Partridge L, Barbas C, Burton D. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci U S A. 1993;90:4141–5. DOIPubMedGoogle Scholar
- Vaughan T, Williams A, Pritchard K, Osbourn J, Pope A, Earnshaw J, Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–14. DOIPubMedGoogle Scholar
- Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7:13–21. DOIPubMedGoogle Scholar
- Mendez MJ, Green LL, Corvalan JR, Jia XC, Maynard-Currie CE, Yang XD, Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15:146–56. DOIPubMedGoogle Scholar
- Sherman-Gold R. Monoclonal antibodies: the evolution from '80s magic bullets to mature, mainstream applications as clinical therapeutics. Genetic Engineering News 1997:17.
- Vaughan TJ, Osbourn JK, Tempest PR. Human antibodies by design. Nat Biotechnol. 1998;16:535–9. DOIPubMedGoogle Scholar
- Barbas C, Hu D, Dunlop N, Sawyer L, Cababa D, Hendry R, In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci U S A. 1994;91:3809–13. DOIPubMedGoogle Scholar
- Barbas CF, Burton DR. Selection and evolution of high-affinity human anti-viral antibodies. Trends Biotechnol. 1996;14:230–4. DOIPubMedGoogle Scholar
- Crothers D, Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972;9:341–57. DOIPubMedGoogle Scholar
- Sherwood JK, Zeitlin L, Chen X, Whaley KJ, Cone RA, Saltzman WM. Residence half-life of IgG administered topically to the mouse vagina. Biol Reprod. 1996;54:264–9. DOIPubMedGoogle Scholar
- Glassy M. Production: the rate-limiting step in obtaining human monoclonal antibody pharmaceuticals. In: Monoclonal antibody production. La Jolla (CA): International Business Communications; 1996.
- DeYoung G. Monoclonal Ab processors/manufacturers stress costs and productivity. Genetic Engineering News 1996:8.
- Hiatt A, Caffertey R, Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342:76–8. DOIPubMedGoogle Scholar
- Genzyme transgenic manufactures monoclonal antibody in goats' milk [press release]. Cambridge (MA): Genzyme; May, 1995.
- Seaver S. Monoclonal antibodies: using new techniques to reduce development time. Genetic Engineering News 1997:13.
- Food and Drug Administration. International conference on harmonisation; guidance on preclinical safety evaluation of biotechnology-derived pharmaceuticals; availablity. Fed Regist. 1997;62:61515–9.
- OraVax Reports Results from Phase III Trial of HNK20 Nosedrop for Respiratory Syncytial Virus in Infants [press release]. Cambridge (MA): OraVax; March 19, 1997.
- Ma J, Smith R, Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987;55:1274–8.PubMedGoogle Scholar
- Beck L, Boots L, Stevens V. Absorption of antibodies from the baboon vagina. Biol Reprod. 1975;13:10–6. DOIPubMedGoogle Scholar
- Corthesy B, Kaufmann M, Phalipon A, Peitsch M, Neutra M, Kraehenbuhl J-P. A pathogen-specific epitope inserted into recombinant secretory immunoglobulin A is immunogenic by the oral route. J Biol Chem. 1996;271:33670–7. DOIPubMedGoogle Scholar
- Tsume Y, Taki Y, Sakane T, Nadai T, Sezaki H, Watabe K, Quantitative evaluation of the gastrointestinal absorption of protein into the blood and lymph circulation. Biol Pharm Bull. 1996;19:1332–7.PubMedGoogle Scholar
- Kuo PY, Sherwood JK, Saltzman WM. Topical antibody delivery systems produce sustained levels in mucosal tissue and blood. Nat Biotechnol. 1998;16:163–7. DOIPubMedGoogle Scholar
- Points to consider in the manufacture and testing of monoclonal antibody products for human use. Washington: U.S. Department of Health and Human Services, Food and Drug Administration; 1997.
- Ma JK, Hein MB. Plant antibodies for immunotherapy. Plant Physiol. 1995;109:341–6. DOIPubMedGoogle Scholar
- Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–6. DOIPubMedGoogle Scholar
- Mo H, Stamatatos L, Ip J, Barbas C, Parren P, Burton D, Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–74.PubMedGoogle Scholar
- Babcook JS, Leslie KB, Olsen OA, Salmon RA, Schrader JW. A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc Natl Acad Sci U S A. 1996;93:7843–8. DOIPubMedGoogle Scholar
- Murray C, Lopez A. Global and regional cause-of-death patterns in 1990. In: Murray C, Lopez A, editors. Global comparative assessments in the health sector. Geneva: World Health Organization; 1994. p. 21-54.
- Eng T, Butler W. The hidden epidemic. Institute of Medicine. Washington: National Academy Press; 1997.
- Bogstedt A, Johansen K, Hatta H, Kim M, Casswall T, Svensson L, Passive immunity against diarrhoea. Acta Paediatr. 1996;85:125–8. DOIPubMedGoogle Scholar
- Eibl M, Wolf H, Furnkranz H, Rosenkranz A. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA-IgA feeding. N Engl J Med. 1988;319:1–7.PubMedGoogle Scholar
- Petschow B, Talbott R. Reduction in virus-neutralizing activity of a bovine colostrum immunoglobulin concentrate by gastric acid and digestive enzymes. J Pediatr Gastroenterol Nutr. 1994;19:228–35. DOIPubMedGoogle Scholar
- Ogra P, Fishaut M. Human breast milk. In: Remington J, Klein J, editors. Infectious diseases of the fetus and newborn infant. 3rd ed. Philadelphia: W.B. Saunders Company; 1990. p. 68-84.
- Prince G, Hemming V, Horswood R, Baron P, Chanock R. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. 1987;61:1851–4.PubMedGoogle Scholar
- Radomsky ML, Whaley KJ, Cone RA, Saltzman WM. Controlled vaginal delivery of antibodies in the mouse. Biol Reprod. 1992;47:133–40. DOIPubMedGoogle Scholar
- Sherwood J, Zeitlin L, Whaley K, Cone R, Saltzman W. Controlled release of antibodies for long-term topical passive immunoprotection of female mice against genital herpes. Nat Biotechnol. 1996;14:468–71. DOIPubMedGoogle Scholar
- Tjokronegoro A, Sirisinha S. Degradation of immunoglobulins by secretions of human reproductive tracts. J Reprod Fertil. 1974;38:221–4. DOIPubMedGoogle Scholar
- Singh M, Sugathan PS, Bhujwala RA. Human colostrum for prophylaxis against sticky eyes and conjunctivitis in the newborn. J Trop Pediatr. 1982;28:35–7.PubMedGoogle Scholar