Volume 6, Number 2—April 2000
Dispatch
Chlamydia pneumoniae Infection in a Breeding Colony of African Clawed Frogs (Xenopus tropicalis)
Abstract
More than 90% of a breeding colony of clawed frogs (Xenopus tropicalis) imported to the United States from western Africa died in an epizootic of chlamydiosis. Chlamydial inclusions were observed by light and electron microscopy in the liver of an infected frog. Chlamydia pneumoniae was isolated in cell cultures from four frogs. A cutaneous infection by a chytridiomycete fungus observed in two frogs could have been a cofactor in the die-off.
Chlamydia infections cause disease in humans, birds, and mammals. Of the four currently recognized Chlamydia species, C. psittaci is the most important animal pathogen. Psittacosis, which can manifest as severe enteric and respiratory illness in many avian species, is highly contagious and can be transmitted to humans and many other mammals (1). C. pecorum, the newest species to be recognized, appears to have a highly restricted host range. Infections due to C. pecorum have been associated with sporadic encephalitis, polyarthritis, pneumonia, and conjunctivitis in pigs, sheep, cattle, and koalas (2,3). C. trachomatis, an agent responsible for millions of cases of ocular and urogenital infections worldwide, causes most chlamydial infections in humans. C. pneumoniae, an acute respiratory tract pathogen of cosmopolitan distribution, may be linked with chronic diseases such as coronary atherosclerosis and multiple sclerosis (1,4,5).
Initial reports of infections due to C. pneumoniae suggested that the organism's host range was limited to humans. Subsequently, infections due to C. pneumoniae have been documented in koalas, a horse, and most recently, a giant barred frog (Mixophyes iteratus) from Australia (6-8). We report an epizootic of chlamydiosis due to C. pneumoniae in a commercial colony of African clawed frogs (Xenopus tropicalis).
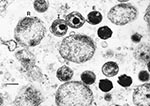
In July 1998, a biologic supply company located in the midwestern United States imported 241 X. tropicalis from western Africa to start a breeding colony. Upon arrival, the frogs were placed in quarantine. Within 4 months, 220 (91%) of the frogs had died. Before death, most of the frogs had sloughing of the skin and lethargy and appeared bloated. Two frogs were submitted to our veterinary diagnostic laboratory for necropsy. On external examination, the frogs were 5.5 cm and 5.2 cm from snout to vent and weighed 6.5 gm and 29.5 gm, respectively. Both frogs had roughening and early sloughing of the posterior skin. The smaller frog had scattered subserosal intestinal and hepatic nodules containing tetrathrydian cestodes. The larger frog was extremely edematous, with clear gelatinous fluid in the subcutaneous tissues and body cavities. In hematoxylin- and eosin-stained 4-µm sections of liver from the larger frog, there was moderate to severe lymphohistiocytic hepatitis with individual hepatocyte necrosis, fibrosis, and numerous granular basophilic bodies resembling bacteria within hepatocytes and histiocytes. Transmission electron microscopy of liver tissue from the larger frog confirmed the presence of bacteria replicating within vacuoles of infected cells. The organisms had a biphasic structure consistent with a Chlamydia spp. (Figure). Other lesions present in both frogs included mild to moderate lymphohistiocytic interstitial pneumonia and mild lymphocytic interstitial nephritis. No chlamydial inclusions were observed in other organs by light microscopy. Both frogs had multifocal epidermal hyperplasia with numerous intracorneal fungal organisms characterized by thick-walled sporangia containing multiple 2-μm to 3-μm round spores. Many of the sporangia had single tubular extensions (discharge papillae) directed toward the skin surface, and transmission electron microscopy showed the spores had flagella. These features were considered diagnostic of a chytridiomycete fungus (9).
To further characterize the Chlamydia species, liver samples were submitted for culture from the larger frog and three additional frogs obtained subsequently from the same breeding colony. Tissue homogenates were injected into shell-vials having monolayers of either buffalo green monkey kidney (BGMK), Hep-2, McCoy, or HeLa - 229 cells. Cultures were incubated at 35C for 72 hours and then the cell monolayers were stained with a fluorescein-conjugated monoclonal antibody specific for the genus Chlamydia (Pathfinder, Kallestad, California). Chlamydia grew from all four liver specimens and in all the cell lines used. The organism grew better, with more and larger inclusions, in the McCoy and BGMK cell lines.
Chlamydia was identified by sequence analysis of the 16S rDNA and major outer membrane protein A (ompA) genes. Bacterial DNA was extracted by lysis (Generations, Gentra, Maryland) of liver and cell culture specimens from each of the four frogs. The broad-range eubacterial primers FD1(5'-AGAGTTTGATCCTGGCTCAG-3') and RD1(5'-AAGGAGGTGATCCAGCC-3') were used in a polymerase chain reaction (PCR) to amplify a 1,520-bp segment of the 16S rDNA gene (10); primers 20CHOMP(5'-TTAGAGGTRAGWATGAARAA-3') and CHOMP371 (5'-TTAGAAICKGAATTGIGCRTTIAYGTGIGCIGC-3') were used to amplify a 1,200-bp segment of the ompA gene (11). Agarose gel electrophoresis of the PCR products yielded single bands for each specimen. Amplified products were column purified to remove unincorporated primers and nucleotides, and sense and antisense strands were sequenced by using nested primers in an automated laser fluorescent sequencer (ALF Express, Amersham Pharmacia Biotech, New Jersey). Sequence fragments were aligned with the assembly software program DNAsis 2.5 (Hitachi, California), and ambiguities resolved. A contiguous sequence was established from the overlapping regions, which were used as a query sequence in the GenBank database.
The 1,520-bp 16S rDNA sequences from the Chlamydia infecting the four X. tropicalis (GenBank-AF139200) were identical to one another and had 99.1%, 99.3%, and 99.4% homology with published sequences for C. pneumoniae strains N16 (U68426.2), CWL026 (AE001668), and TW-183 (L06108), respectively. In addition, there was 100% homology with the 235-bp segment of 16S rDNA reported for the C. pneumoniae isolated recently from the giant barred frog from Australia (AF102832). In contrast, there was 96.0% sequence homology with C. psittaci strain 6BC (U68447.2), 95.7% homology with C. pecorum strain E58 (D88317.1), and 93.8% homology with C. trachomatis strain D/UW-3/CX (AE001345).
The sequence of a 279-bp segment of the variable domain IV region of the ompA gene was determined for one of the frog Chlamydia isolates (AF184214). There were six nucleotide differences (97.8% homology) from the giant barred frog biovar of C. pneumoniae (AF102830) and three differences (98.9% homology) from C. pneumoniae strain CWL029 (AE001652). Less than 75% homology was observed with the ompA variable domain IV sequences reported for the three other Chlamydia species (results not shown).
Previous reports of confirmed Chlamydia infections among captive amphibians in North America involved X. laevis, another member of the African clawed frog family (12-14). These reports occurred before the discovery and molecular characterization of C. pneumoniae, and the Chlamydia species involved were either not known or presumed to be C. psittaci. Our study is the second documented report of C. pneumoniae involving an amphibian and the first describing an epizootic in a captive population of X. tropicalis.
The spectrum of clinical disease and histopathologic lesions associated with chlamydial infections in amphibians is unknown. In our study, only two animals were evaluated histologically. The more severely affected animal had active hepatitis with hepatocyte necrosis, lymphohistiocytic infiltrates, and inclusion bodies within hepatocytes and sinusoidal histiocytes. These lesions are similar to those observed in a 1984 outbreak of chlamydiosis among laboratory-bred X. laevis; in that outbreak, hepatosplenomegaly and histologic evidence of active hepatitis were the major pathologic findings in affected animals (12). In the recent case of C. pneumoniae involving a free-ranging giant barred frog, the predominant necropsy findings were chronic mononuclear pneumonia, nonregenerative anemia, and pancytopenia (8). Additional studies will be necessary to determine the epidemiology and virulence of C. pneumoniae in captive and free-ranging populations of amphibians and reptiles, as well as the pathogenicity and transmissibility of this agent to humans and other mammals.
This breeding colony had >90% deaths during the 4-month epizootic, and the few surviving frogs appeared ill and were subsequently euthanized. We were unable to determine the prevalence of C. pneumoniae infection in the colony but believe that it was very high, since all four frogs submitted for culture were positive. The crowding and stress associated with the captive environment may have contributed to spread of infection within the colony. The potential for zoonotic transmission of C. pneumoniae from amphibians to humans has important public health implications, especially in situations involving frequent and prolonged human contact with amphibians in crowded conditions, such as commercial breeding colonies or zoos. Until more is known about the host range of this pathogen, chlamydiosis should at least be considered in the differential diagnosis of an unexplained respiratory or febrile illness in persons exposed to amphibians.
Whether Chlamydia was the primary pathogen responsible for the die-off or served as a cofactor with the chytrid fungus or other parasites was difficult to determine. Although the Chlamydia infection is the primary focus of this report, the presence of cutaneous chytridiomycosis in two of the frogs is also important. This highly pathogenic zoosporic fungus has only been found in the American and Australian continents, where it has been implicated as a major factor in amphibian deaths and population declines in the rain forests (9). The origin of the chytrid fungus found in our cases was not determined. However, the rapid onset of illness affecting the frogs after their arrival in the United States suggests that at least some of the frogs may have been infected before capture.
The pipid frog X. tropicalis has a diploid genome and short generation time, which make it an ideal model organism for multigenerational genetic analysis. Current demand for this species is higher than most biologic supply companies can meet. Continued importation of X. tropicalis and other amphibians from regions of the world experiencing poorly understood population declines raises concern about the inadvertent spread of virulent pathogens to naive populations of amphibians and reptiles, as well as transmission of these agents to mammals. Until more is known about the epidemiology and prevention of these infections, caution must be exercised in transportation, husbandry, and human contact with these animals.
Dr. Reed is a clinical pathologist and director of Clinical Research at the Marshfield Medical Research Foundation. His research focuses on the diagnosis and molecular epidemiology of tickborne and other zoonotic pathogens.
Acknowledgments
The authors thank Lynn Ivacic for expert technical assistance with the chlamydial cultures, Donald Stoiber for preparing the electron micrographs, and the veterinary pathology section of the Armed Forces Institute of Pathology for identifying the cestodes and reviewing the histopathology.
This work was supported in part by grants from the Marshfield Medical Research Foundation. Jeanine Meyer was an undergraduate research fellow of Marshfield Clinic.
References
- Schacter J. Infection and disease epidemiology. In: Chlamydia: intracellular biology, pathogenesis, and immunity. Stephens RS, editor. Washington: American Society for Microbiology Press; 1999. p. 139-69.
- Fukushi H, Hirai K. Proposal of Chlamydia pecorum sp. nov. for Chlamydia strains derived from ruminants. Int J Syst Bacteriol. 1992;42:306–9. DOIPubMedGoogle Scholar
- Glassick TV, Giffard P, Timms P. Outer membrane protein 2 gene sequence indicates that Chlamydia pecorum and Chlamydia pneumoniae, cause infection in koalas. Syst Appl Microbiol. 1996;19:457–64.
- Kuo C, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995;8:451–61.PubMedGoogle Scholar
- Campbell LA, Kuo C, Grayston JT. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–9. DOIPubMedGoogle Scholar
- Jackson M, White N, Gifford P, Timms P. Epizootic of Chlamydia infection in two free-ranging Koala populations. Vet Microbiol. 1999;65:255–64. DOIPubMedGoogle Scholar
- Storey C, Lusher M, Yates P, Richmond S. Evidence for Chlamydia pneumoniae of non-human origin. J Gen Microbiol. 1993;139:2621–6.PubMedGoogle Scholar
- Berger L, Volp K, Mathews S, Speare R, Timms P. Chlamydia pneumoniae in a free-ranging giant barred frog (Mixophyes iteratus) from Australia. J Clin Microbiol. 1999;37:2378–80.PubMedGoogle Scholar
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998;95:9031–6. DOIPubMedGoogle Scholar
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703.PubMedGoogle Scholar
- Kaltenboeck B, Kousoulas KG, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of four chlamydial species. J Bacteriol. 1993;175:487–502.PubMedGoogle Scholar
- Howerth EW. Pathology of naturally occurring chlamydiosis in African clawed frogs (Xenopus laevis). Vet Pathol. 1984;21:28–32.PubMedGoogle Scholar
- Newcomer CE, Anver MR, Simmons JL, Wilcke BW, Nace GW. Spontaneous and experimental infections of Xenopus laevis with Chlamydia psittaci. Lab Anim Sci. 1982;32:680–6.PubMedGoogle Scholar
- Wilcke BW, Newcomer CE, Anver MR, Simmons JL, Nace GW. Isolation of Chlamydia psittaci from naturally infected African clawed frogs (Xenopus laevis). Infect Immun. 1983;41:789–94.PubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 6, Number 2—April 2000
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kurt D. Reed, Clinical Research Department, Marshfield Medical Research Foundation, 1000 N. Oak Avenue, Marshfield, WI 54449, USA; fax:715-389-4145
Top