Volume 6, Number 5—October 2000
Research
Naturally Occurring Ehrlichia chaffeensis Infection in Coyotes from Oklahoma
Abstract
A nested polymerase chain reaction assay was used to determine the presence of Ehrlichia chaffeensis, E. canis, and E. ewingii DNA in blood samples of free-ranging coyotes from central and north-central Oklahoma. Of the 21 coyotes examined, 15 (71%) were positive for E. chaffeensis DNA; none was positive for E. canis or E. ewingii. Results suggest that E. chaffeensis infections are common in free-ranging coyotes in Oklahoma and that these wild canids could play a role in the epidemiology of human monocytotropic ehrlichiosis.
Human monocytotropic ehrlichiosis, a tick-borne zoonosis caused by the rickettsial pathogen Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae), occurs primarily in the southern, south-central, and mid-Atlantic regions of the United States (1,2). The principal vector is the lone star tick, Amblyomma americanum (L.), and associations between the presence of the tick and the occurrence of human ehrlichiosis have been documented (1). The principal wildlife reservoir is the white-tailed deer (Odocoileus virginianus) (3,4). Indeed, site-specific geographic and temporal associations have been made between the presence of A. americanum and E. chaffeensis antibodies in deer (5,6). No other wildlife species has been incriminated in the epidemiology of this disease, although serologic reactivity was detected in free-ranging raccoons (Procyon lotor) and opossums (Didelphis virginianus) from Georgia (5) and white-footed mice (Peromyscus leucopus) from Connecticut (7). Additionally, red foxes (Vulpes vulpes) have been shown to be susceptible to infection under experimental conditions (8). Although some rodents have been experimentally infected with this pathogen (9), research findings about natural infections in wild rodent populations have been inconsistent (7,10). Domestic dogs are susceptible to both natural and experimental E. chaffeensis infections (11–13).
To determine whether free-ranging coyotes (Canis latrans) serve as a reservoir host for E. chaffeensis, E. canis, or E. ewingii, we used a nested polymerase chain (PCR) assay to survey for the presence of DNA of these organisms in blood samples from 21 free-ranging coyotes from central and north-central Oklahoma. Coyotes were obtained as part of animal damage control (U.S. Department of Agriculture) from an area in the established range of A. americanum (14,15), in which E. chaffeensis was endemic in deer and E. chaffeensis, E. canis, and E. ewingii had been found in dogs (13,16). Immediately after the coyotes were shot, EDTA-anticoagulated whole blood was collected for isolation of DNA for PCR assay. Blood samples were stored at 4oC until processing. DNA was isolated from whole blood (200 l) with the QIAamp Blood Kit (Qiagen, Santa Clarita, CA), according to the manufacturer's instructions.
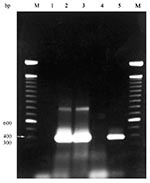
Purified DNA from each blood sample was tested in four PCR amplifications by using primers HE1, HE3, EE5, and ECAN5 (12,13,17); reaction conditions are described in the Figure. For DNA sequencing, PCR reactions were performed, and products were separated by agarose gel electrophoresis. Bands were stabbed multiple times with sterile pipet tips, which were placed into PCR reaction mix as template (19). PCR reactions were pooled and purified by using Qiagen Qiaquick PCR purification kit, according to manufacturer's instructions. DNA was sequenced at the Oklahoma State University Recombinant/DNA Protein Research Facility (Stillwater, OK) using an Applied Biosystems (Foster City, CA) 373A automated DNA sequencer. Sequences were analyzed with MacVector software (Oxford Molecular Group, Inc., Campbell, CA). A partial sequence (300 bp) from each end of the 390-bp amplified fragment was determined for both the E. chaffeensis-positive control and one positive coyote. The sequences obtained here were compared to those previously deposited in GenBank (13) to verify that E. chaffeensis DNA was amplified.
Of the 21 coyotes tested, 15 were positive by PCR assay for E. chaffeensis (Figure); none was positive for E. canis or E. ewingii. To our knowledge, this is the first reported evidence of natural E. chaffeensis infection in a coyote and the first PCR-based evidence in a free-ranging mammal other than white-tailed deer. Although these findings do not question the importance of white-tailed deer in the endemic maintenance of E. chaffeensis, they do point to coyotes as potential reservoir hosts.
All stages of A. americanum feed readily on coyotes (14,20). Moreover, white-tailed deer and coyote populations overlap in much of the E. chaffeensis-A. americanum disease-endemic regions of the United States (1,21–24). Movement of these deer, as indicated by their home range (usually not exceeding 1.6 km [25]) is more restricted than that of coyotes (whose range may exceed 31 km [22]). These behavioral factors, and coyotes’ apparent susceptibility to infection with E. chaffeensis, make them an ideal bridge species for the spread of this tickborne pathogen among wild species as well as a source of infection for ticks that may subsequently feed on other hosts, including humans and domestic animals.
The results of this study, although based on a limited number of free-ranging coyotes, suggest that in the geographic range of the study, coyotes likely play little or no role in the endemic maintenance or spread of other species of Ehrlichia that commonly parasitize domestic dogs or humans. Coyotes are susceptible to experimental infection with E. canis (26), and domestic dogs and ticks from Oklahoma have been shown to be naturally infected with both E. canis and E. ewingii as well as E. chaffeensis (13). In fact, E. ewingii DNA was recently identified from patients in Missouri, which expands the known host range of this organism, making it a newly emerging zoonosis of public health concern (27).
The occurrence of E. ewingii in domestic dogs and ticks in Oklahoma (13), the broad host range of A. americanum (its natural vector [14,23,28]), and the documented occurrence of A. americanum in both wild and domestic canids (13,20,24) suggest a potential for future cross-species transmission of this organism from domestic to wild and human hosts.
Dr. Kocan is a professor in the Department of Veterinary Pathobiology at Oklahoma State University, College of Veterinary Medicine. His research interests include infectious and parasitic diseases of free-ranging wild animals and vector-borne diseases of wild, domestic, and human hosts.
Acknowledgments
We thank J. Lilley and D. Alley for their assistance in obtaining coyotes.
This research was supported in part by Animal Health Grant No. 1433 and project 3714 of the Agricultural Experiment Station and the College of Veterinary Medicine, Oklahoma State University.
References
- Eng TR, Harkess JR, Fishbein DB, Dawson JE, Greene CN, Rredus MA, Epidemiologic, clinical and laboratory findings of human ehrlichiosis in the United States,1988. JAMA. 1990;264:2251–8. DOIPubMedGoogle Scholar
- Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht DE, Shaddock J, White-tailed deer as a potential reservoir for Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Wildl Dis. 1994;30:162–8.PubMedGoogle Scholar
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:368–74.PubMedGoogle Scholar
- Lockhart JM, Davidson WR, Dawson JE, Stallknecht DE, Howerth EW. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–6.PubMedGoogle Scholar
- Lockhart JM, Davidson WR, Dawson JE, Stallknecht DE, Little SE. Natural history of Ehrlichia chaffeensis in the Piedmont physiographic province of Georgia. J Parasitol. 1997;83:887–94. DOIPubMedGoogle Scholar
- Lockhart JM, Davidson WR, Dawson JE, Stallknecht DE. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J Wildl Dis. 1995;31:119–24.PubMedGoogle Scholar
- Magnarelli LA, Anderson AF, Stafford KC, Dumler JS. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. J Wildl Dis. 1997;33:466–73.PubMedGoogle Scholar
- Davidson WR, Lockhart JM, Stallknecht DE, Howerth EW. Susceptibility of red and gray foxes to infection by Ehrlichia chaffeensis. J Wildl Dis. 1999;35:696–702.PubMedGoogle Scholar
- Telford SR, Dawson JE. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet Microbiol. 1996;52:103–12. DOIPubMedGoogle Scholar
- Lockhart JM, Davidson WR, Stallknecht DR, Dawson JE. Lack of seroreactivity to Ehrlichia chaffeensis among rodent populations. J Wildl Dis. 1998;34:392–6.PubMedGoogle Scholar
- Dawson JE, Ewing SA. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322–7.PubMedGoogle Scholar
- Dawson JE, Biggie KL, Warner CK, Cookson K, Jenkens S, Levine JF, Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–9.PubMedGoogle Scholar
- Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–39. DOIPubMedGoogle Scholar
- Hair JA, Bowman JL. Behavioral ecology of Amblyomma americanum (L.). In: Sauer J, Hair JA, editors. Morphology, physiology, and behavioral biology of ticks. New York: Ellis Horwood Limited;1986. p. 406-27.
- Cooley RA, Kohls GM. The genus Amblyomma (Ixodidae) in the United States. J Parasitol. 1944;30:77–111. DOIGoogle Scholar
- Dawson JE, Warner CK, Baker V, Ewing SA, Stallknecht DE, Davidson WR, Ehrlichia-like 16S rDNA from wild white-tailed deer (Odocoileus virginianus). J Parasitol. 1996;82:52–8. DOIPubMedGoogle Scholar
- Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Green CR, Olson JG, Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–80.PubMedGoogle Scholar
- Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, . Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–8.PubMedGoogle Scholar
- Wilton SD, Lim L, Dye D, Laing N. Bandstab: a PCR-based alternative to cloning PCR products. Biotechniques. 1997;22:642–5.PubMedGoogle Scholar
- Kocan AA, Breshears M, Cummings C, Panciera RJ, Ewing SA, Barker RW. Naturally occurring hepatozoonosis in coyotes from Oklahoma. J Wildl Dis. 1999;35:86–9.PubMedGoogle Scholar
- Baker RH. Origin, classification, and distribution. In: Halls L, editor. White-tailed deer. ecology and management. Harrisburg, PA: Stackpole Books;1984. p. 1-18.
- Nowak RM. Walker's mammals of the world (5th ed). Baltimore: The John Hopkins University Press;1991. vol. 2. p. 1068-70.
- Patrick CD, Hair JA. White-tailed deer utilization of three different habitats and its influence on lone star tick populations. J Parasitol. 1978;64:1100–6. DOIGoogle Scholar
- Bloemer SR, Zimmerman RH. Ixodid ticks on the coyote and gray fox at Land-Between-the-Lakes, Kentucky-Tennessee, and implications for tick dispersal. J Med Entomol. 1988;25:5–8.PubMedGoogle Scholar
- Morchonton RL, Hirth DH. Behavior. In: Halls L, editor. White-tailed deer: ecology and management. Harrisburg, PA: Stackpole Books;1984. p. 129-68.
- Ewing SA, Buckner RG, Stringer BG. The coyote, a potential host for Babesia canis and Ehrlichia sp. J Parasitol. 1964;50:704. DOIPubMedGoogle Scholar
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–55. DOIPubMedGoogle Scholar
- Anziani OS, Ewing SA, Barker RW. Experimental transmission of a granulocytic form of the Tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res. 1990;51:929–31.PubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 6, Number 5—October 2000
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
A. Alan Kocan, Department of Veterinary Pathobiology, College of Veterinary Medicine, Oklahoma State University, Stillwater, OK 74078; fax: 407-744-8263
Top