Volume 7, Number 4—August 2001
THEME ISSUE
West Nile Virus
West Nile Virus
Widespread West Nile Virus Activity, Eastern United States, 2000
Abstract
In 1999, the U.S. West Nile (WN) virus epidemic was preceded by widespread reports of avian deaths. In 2000, ArboNET, a cooperative WN virus surveillance system, was implemented to monitor the sentinel epizootic that precedes human infection. This report summarizes 2000 surveillance data, documents widespread virus activity in 2000, and demonstrates the utility of monitoring virus activity in animals to identify human risk for infection.
In August and September 1999, an epidemic of encephalitis and aseptic meningitis caused by West Nile (WN) virus occurred in New York City (1-3). This epidemic was preceded by anecdotal reports of an extensive die-off among American Crows (Corvus brachyrhynchos) and several other bird species in the most affected boroughs of New York City (1-3). The WN virus epidemic in the northeastern United States in 1999 underscores the ease with which an emerging arthropod-borne flavivirus and human pathogen can become established in a new geographic area. In addition, the occurrence of a widespread epizootic as a sentinel event that precedes human infection emphasizes the importance of establishing ecologic surveillance to identify conditions that might result in human infections.
In 1999, establishment of enhanced human and animal infection surveillance was recommended in states either affected in 1999 or at higher risk for becoming affected because of bird migration patterns (4). New York City, the District of Columbia, 20 states along the Atlantic and Gulf coasts, and the Centers for Disease Control and Prevention (CDC) developed and implemented ArboNET, a cooperative WN virus surveillance system designed to provide data to monitor the geographic and temporal spread of WN virus in the United States; to identify areas at increased risk for human infections with WN virus; to develop strategies to prevent WN virus infections in humans or animals or to minimize the number of these infections once an outbreak occurs; and to determine the distribution and incidence of the other domestic arboviruses.
To accomplish these goals, cooperating jurisdictions performed the following surveillance activities: bird surveillance monitoring, including deaths and seroprevalence among wild birds and seroconversion among sentinel chicken flocks; mosquito surveillance; enhanced equine and nonhuman mammal surveillance; and enhanced passive or active human surveillance (5). The same system collected data regarding confirmed and probable WN virus-infected humans, nonhuman vertebrates, and mosquitoes, in addition to the number of specimens from each species that were collected and tested.
This report summarizes the findings of surveillance data collected in 2000, which document widespread WN virus activity throughout the eastern United States and the utility of monitoring WN virus activity in birds and mosquitoes to identify areas at increased risk for human infection.
This summary includes surveillance data for 2000 that were collected from 20 states (Alabama, Connecticut, Delaware, Florida, Georgia, Louisiana, Maine, Maryland, Massachusetts, Mississippi, New Hampshire, New Jersey, New York, North Carolina, Pennsylvania, Rhode Island, South Carolina, Texas, Vermont, and Virginia), New York City, and the District of Columbia. All states began to submit surveillance data for May 2000 except New York (started with January 2000 data), Vermont (started with June 2000 data), and New Hampshire (started with July 2000 data). Except for Louisiana, New Hampshire, and Maine, which stopped submitting data in October 2000, all other states collected data at least through mid-November 2000.
Data about surveillance activities were gathered by counties in these 20 states and forwarded to a state WN virus surveillance coordinator. At the state level, data aggregated by county and by week of bird report, specimen collection, or illness onset were entered into a standardized database and electronically reported to CDC weekly. Types of data included the numbers of dead crows and dead birds of other species reported by county residents; crows and birds of other species that were tested for evidence of WN virus infection; mosquitoes of a specific species that had been collected; wild birds that were trapped and bled to determine the prevalence of recently developed antibody against WN virus; sentinel chickens that had been bled to identify seroconversion following recent WN virus infection; and ill or dead humans, horses, and other mammals from which a tissue or serum sample had been submitted to determine if illness or death was attributable to WN virus infection.
In addition, humans, nonhuman vertebrates, and mosquitoes with documented WN virus infections were reported continuously to CDC by telephone, facsimile, or e-mail from the 20 states, New York City, and the District of Columbia. Reports were submitted either directly from the state public health laboratory or the WN virus surveillance group. The methods used to document infection differed by state, species, and the type of tissue tested (5). In mosquitoes and nonhuman vertebrates, testing included combinations of reverse-transcription polymerase chain reaction or real-time (TaqMan) polymerase chain reaction to identify WN virus genome in tissue or cerebrospinal fluid (CSF); immunofluorescent or immunohistochemistry studies to demonstrate WN virus antigen in tissue; virus culture from tissue or serum; or serology testing using immunoglobulin (Ig) M-capture enzyme-linked immunosorbent assay (MAC-ELISA) or plaque-reduction neutralization test (PRNT) to identify WN virus-specific antibodies that demonstrate recent infection. In ill humans, WN virus infections were confirmed by isolating WN virus from or demonstrating WN viral antigen or genomic sequences in tissue, blood, CSF, or other body fluid; demonstrating IgM antibody to WN virus in CSF by MAC-ELISA; demonstrating a fourfold serial change in PRNT antibody titer to WN virus in paired, appropriately timed serum samples; or demonstrating both WN virus-specific IgM by MAC-ELISA and IgG antibody in a single serum specimen by various methods. The county, state, specific species, and the week of bird report, specimen collection, or illness onset that corresponded to each reported WN virus-infected human, nonhuman vertebrates, or mosquito were also collected.
Humans
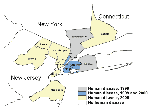
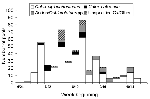
In 2000, 21 persons in the northeastern United States were reported with acute illness attributed to WN virus infection; 19 were hospitalized with severe neurologic illness (12 with encephalitis, 4 with meningitis, and 3 with meningoencephalitis). Of the 19 hospitalized patients, 2 (11%) died. Of the 21 patients, 10 lived in the Staten Island Borough (Richmond County) of New York City (Figure 1). Other patients lived in nine other counties: Kings (Brooklyn), New York (Manhattan), and Queens counties in New York; Hudson, Passaic, Monmouth, Morris, and Bergen counties in New Jersey; and Fairfield County in Connecticut. Patients were 36 to 87 years of age (median 62 years); 13 (62%) were men. Dates of illness onset were from July 20 to September 27 (Figure 2). The peak incidence occurred the week starting August 26, during which five WN virus-infected persons had onset of illness.
Ecologic Surveillance and Human Illness
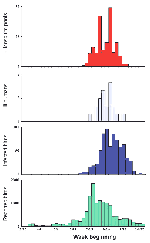
In all 10 counties subsequently reporting human cases in 2000, a WN virus-infected bird was found an average of 44 days (range 15 to 92 days) before the illness onset date of the first human case (Table 1). In 8 of the 10 counties, infected mosquito pools were collected an average of 32 days (4 to 54 days) before the illness onset date. In the other two counties, no infected mosquito pools were found in 2000 despite intensive collection efforts. Similarly, in the 10 counties that reported human illnesses caused by WN virus infection, the number of dead and ill birds reported by residents increased many weeks before the first human cases (Figure 2).
Crows and Other Birds
In 2000, residents in 321 counties in 16 states reported at least one dead bird to their local or state health department, for a total of 104,816 dead birds (30,601 crows and 74,215 other birds). Of these 104,816 reported birds, 12,961 (12.4%) were submitted for WN virus testing; 4,305 (33.2%) were WN virus infected. Of the 7,580 crows tested, 3,824 (50.4%) were infected, compared with 481 (8.9%) of 5,381 birds of other species tested.
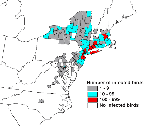
Epizootic activity in birds was widespread (Figure 3). WN virus-infected dead birds were reported from 136 counties in 12 states and the District of Columbia (New York reported 1,263 birds; New Jersey 1,280; Connecticut 1,118; Massachusetts 449; Rhode Island 87; Maryland 50; Pennsylvania 36; New Hampshire 7; Virginia 7; Delaware 1; North Carolina 1; Vermont 1; and the District of Columbia, 5). Crows and related corvid species were the most frequently reported WN virus-infected species. Of the 4,305 reported WN virus-infected birds, 3,824 (88.8%) were Corvus species (American Crow, Fish Crow [C. ossifragus], Common Raven [C. corax]), and 196 (4.6%) Blue Jays (Cyanocitta cristata) (Table 2). The remaining 285 (6.6%) reported, WN-virus-infected birds included 59 other bird species. Dead WN virus-infected birds were found over a 9-month period (from a Red-tailed Hawk [Buteo jamaicensis] found in Westchester County, New York, on February 6 to an American Crow found on November 17 in Barnstable County, Massachusetts). However, of the 4,305 ill or dead birds confirmed to have WN virus infection, 3,637 (84.5%) were found from July 1 through September 30.
Mosquitoes
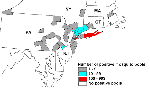
WN virus was isolated from or WN virus gene sequences were detected in 515 mosquito pools in 38 counties in five states: 393 pools in New York, 58 in New Jersey, 46 in Pennsylvania, 14 in Connecticut, and 4 in Massachusetts (Figure 4). Of the infected pools, Culex species accounted for 428 (89.2%), including 228 pools of Cx. pipiens/restuans, 146 of Cx. pipiens, 50 of Cx. salinarus, 12 of Cx. restuans, and 26 unspecified Culex pools (Table 3). Ochlerotatus species (formerly in Aedes genus) accounted for 29 WN virus-infected pools (including 9 of Oc. japonicus, 9 of Oc. triseriatus, and 8 of Oc. trivittatus), and Aedes species accounted for 19 WN virus-positive pools (including 17 pools of Ae. vexans). In 2000, by nucleic acid amplification techniques, WN virus genome was identified in at least one pool of all 14 species. Despite attempts to isolate virus from at least one pool of all 14 species, no viral isolate was obtained from three species (Ae. albopictus, Oc. atropalpus, and Anopheles punctipennis).
For the most commonly identified infected mosquito species, collections during the week beginning August 26 yielded the peak number of WN virus-infected mosquito pools (Figure 5). Of 386 positive pools of Cx. pipiens or Cx. restuans collected during the 2000 transmission season (July 7 to November 4), 63 (16.3%) were collected in this week. Of 50 positive pools of Cx. salinarius collected in 2000, 8 (16%) were collected this week, and of 48 positive pools of Aedes or Ochlerotatus, 11 (23%) were collected this week.
Other Surveillance Components
Veterinary surveillance identified WN-virus infections in 63 horses with neurologic disease from 26 counties in 7 states (28 horses in New Jersey; 21 in New York; 7 in Connecticut; 4 in Delaware; and 1 each in Massachusetts, Pennsylvania, and Rhode Island). Illness onsets were from August 17 to November 1, with a peak of 15 horses with onsets during the week of October 7.
In addition, WN infection was confirmed in six other mammals. Of these, five mammals (big brown bat, Eptesicus fuscus; little brown bat, Myotis lucifugas; eastern chipmunk, Tamias striatus; eastern gray squirrel, Sciurus carolinenesis; and domestic rabbit, Oryctologus cuniculus) were from four counties (Albany, Columbia, Bronx, and Rensselaer) in New York State and one (eastern striped skunk, Mephitis mephitis) was from Fairfield County, Connecticut. All were ill; they were collected from August 31 to September 30.
Seroconversion consistent with recent WN virus infection was documented in 13 sentinel chickens in six counties. In Essex, Sussex, Middlesex, and Morris counties, New Jersey, serum samples were drawn from September 27 to 29; in Westchester and Kings (Brooklyn) counties, New York, samples were collected from August 23 to November 3.
Although WN virus was first identified in metropolitan New York City in 1999, surveillance data submitted to the ArboNET WN virus surveillance system have shown a widespread geographic range of virus activity in 2000. Epizootic activity in birds was reported from nine jurisdictions without recognized WN virus activity in 1999 (District of Columbia, Delaware, Massachusetts, New Hampshire, North Carolina, Pennsylvania, Rhode Island, Vermont, and Virginia), as well as the four states that reported activity in 1999 (Connecticut, Maryland, New Jersey, and New York). Similarly, human illnesses attributable to WN virus infection in 2000 were reported from seven counties without identified human illnesses in 1999, as well as three of the six counties that reported human illnesses in 1999.
Despite the widespread virus activity and regional intensification of surveillance activities, 21 acute human illnesses attributable to WN virus infection were identified in 2000, compared with 62 in 1999. Although some decrease in severe human illness may be attributable to vector control and other prevention activities, experience in Europe shows that incidence of human illness can be variable and outbreaks sporadic. Because widespread WN virus epizootic activity probably will persist and expand in the United States, large outbreaks of illness attributable to WN virus infection are possible if adequate surveillance, prevention activities, and mosquito control are not established and maintained.
The large number of avian deaths, particularly among highly recognizable and common birds such as the American Crow, has provided a unique view of a widespread and possibly expanding epizootic from a newly introduced flavivirus. However, a more important question is to what extent avian deaths and mosquito surveillance can serve as early warning sentinels of epizootic activity, so that increased prevention and intervention activities can be implemented before human infections occur. In 2000, all 21 patients had illness onsets at least 15 days after WN virus-infected birds were first collected in the county of residence, suggesting that avian data may be a sensitive indicator of the level of activity associated with subsequent human disease. However, the occurrence of an infected bird in a county was a relatively poor predictor of human illness. Of 136 counties reporting WN virus-infected birds in 2000, 10 (7%) reported humans with illness due to WN virus infection. Further research to identify threshold levels with greater positive predictive value should be undertaken.
The presence of WN virus-infected mosquito pools may be a less sensitive indicator of epizootic activity associated with subsequent human disease. In 2000, 14 of the 21 patients had illness onsets at least 15 days after WN virus-infected mosquito pools were first collected in their county of residence. However, 8 (21%) of the 38 counties with positive mosquito pools reported at least one ill person. Further analysis of 2000 surveillance data, including an assessment of the timing, number, and geographic location of WN virus-infected birds, and an assessment of mosquito-trapping activities, infection rates, and species identified are required to further interpret these data and refine their use.
The avian deaths and mosquito-based surveillance data from the northeastern United States in 2000 indicate that these surveillance modalities may have greater utility as an early warning system for human infections than surveillance among horses and other nonhuman mammal species. Although documented infections among crows occurred as early as April, most reported WN virus illnesses in horses and small mammals occurred relatively late compared with human illnesses. The horse epizootic peaked 6 weeks later and persisted 5 weeks longer than the human epidemic. Similarly, although few infected small mammals were reported, these also occurred relatively later than human illnesses. More data are needed to determine the reasons for this relative delay in horses and small mammals, and, as the epizootic expands, further evaluation of these surveillance modalities in other regions of North America will be required.
The persistence of widespread WN virus activity in 2000 indicates the need for expanded surveillance and prevention activities. In 2001, enhanced ecologic surveillance should be a high priority for states that have been affected or at high risk for being affected by WN virus (6). States with potential for WN virus activity should establish the following: 1) surveillance systems to receive reports of dead and ill crows and other corvids and to collect and test these reported specimens; 2) rapid mosquito surveillance in response to reports of dead WN virus-infected birds to identify potential mosquito vectors, especially those with a propensity to feed on mammals, and to monitor the population densities of those vectors; and 3) enhanced passive surveillance for neurologic disease in horses and other animals to monitor the degree of WN virus transmission outside the bird-mosquito cycle.
Depending on the geographic location of the state, this surveillance should be implemented in the spring and continued until late fall (for states where mosquito activity will cease because of cold weather) or through the winter (for southern states where mosquito activity may be continuous throughout the year).
Even before the recognition of WN virus activity, prevention activities in these states should include programs to 1) eliminate mosquito-breeding habitats in public areas; 2) control mosquito larvae where these habitats cannot be eliminated; 3) promote the increased use of personal protection and reduce peridomestic conditions that support mosquito breeding; and 4) implement adult mosquito control when indicated by increasing WN virus activity or the occurrence of human disease. In addition, because arbovirus infections are endemic in the United States, jurisdictions should have a comprehensive plan and a functional arbovirus surveillance and response capacity that includes trained personnel with suitable laboratory support for identifying arbovirus activity, including WN virus.
In summary, WN virus activity was widespread and possibly expanding in 2000. Although the coordinated, multistate surveillance effort may have led to a wider recognition of epizootic activity in 2000, reports of equine cases from counties that were not affected in 1999 and the large number of reported and WN virus-infected birds strongly suggest that a true expansion occurred. Because of the success of this system in accomplishing its goals, this coordinated, multistate surveillance effort will be expanded in 2001 to include all the continental United States.
Dr. Marfin is a medical epidemiologist with the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention. His research focuses on the epidemiology of infectious diseases involving the central nervous system, including the mosquito-borne arboviruses.
Acknowledgment
We thank Rob Lanciotti, Nick Komar, Chet Moore, Roger Nasci, and Harry Savage for their many contributions to the epidemiologic activities reported in this paper.
References
- Centers for Disease Control and Prevention. Outbreak of West Nile-like viral encephalitis--New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:845–9.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: West Nile viral encephalitis--New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:944–6, 955.
- Asnis DS, Conetta R, Teixeira AA, Waldmon G, Sampspon BA. The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis. 2000;30:413–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Guidelines for surveillance, prevention, and control of West Nile virus infection. MMWR Morb Mortal Wkly Rep. 2000;49:25–8.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Human West Nile virus surveillance--Connecticut, New Jersey, and New York. MMWR Morb Mortal Wkly Rep. 2001;50:265–8.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Epidemic/epizootic West Nile virus in the United States: Revised Guidelines for Surveillance, Prevention, and Control (2001). Available from: URL: http://www.cdc.gov/ncidod/dvbid/westnile/resources/wnv-guidelines-apr-2001.pdf
Figures
Tables
Cite This Article1Centers for Disease Control and Prevention: Tammie Hilger, John E. Jones, Jennifer A. Lehman, Kimlea Medlin, Tim Morris, Mindy J. Perilla, Suzanne Sutliff, David Withum; New Jersey: Faye Sorhage, Christina Tan; Maine: Geoff Beckett, Kathleen Gensheimer; New Hampshire: Jesse Greenblatt, Jose Montero; Vermont: Peter Galbraith, Patsy Tassler; Massachusetts: Alfred DeMaria, Bela Matyas, Ralph Timperi; Rhode Island: Utpala Bandy, Tara Breslosky; Connecticut: Theodore Andreadis, Matthew Cartter, Tara McCarthy,; New York (state): Bryon Backenson, Yoichiro Hagiwara, Laura Kramer, Dale Morse, Barbara Wallace, Dennis White, Amy Willsey, Susan Wong; New York City: Bryan Cherry, Annie Fine, Jackie Kellachan, Varuni Kulakasera, Iqbal Poshni; Pennsylvania: James Rankin; Delaware: Leroy Hathcock, Dave Wolfe; Maryland: Jeffrey Roche; Virginia: Suzanne Jenkins; Washington, D.C.: Martin Levy; North Carolina: J. Newton MacCormack; South Carolina: Jerry Gibson; Georgia: Paul Blake, Stacey Kramer, Susan Lance-Parker; Florida: Lisa Conti, Richard S. Hopkins, Robin Oliveri; Alabama: J.P. Lofgren, Charles H. Woernle; Mississippi: Mary Currier, Sally Slavinski; Louisiana: Karen Kelso; and Texas: Julie Rawlings.
Table of Contents – Volume 7, Number 4—August 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Anthony A. Marfin, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, P.O. Box 2087, Fort Collins, Colorado 80521, USA; fax: 970-221-6476
Top