Volume 7, Number 6—December 2001
Research
The Serologic Response to Cryptosporidium in HIV-Infected Persons: Implications for Epidemiologic Research
Abstract
Advances in serologic assays for Cryptosporidium parvum have made serology an attractive surveillance tool. The sensitivity, specificity, and predictive value of these new assays for surveillance of immunocompromised populations, however, have not been reported. Using stored serum specimens collected for the San Francisco Men's Health Study, we conducted a case-control study with 11 clinically confirmed cases of cryptosporidiosis. Based on assays using a 27-kDa antigen (CP23), the serum specimens from cases had a median response immunoglobulin (Ig) G level following clinical diagnosis (1,334) and a net response (433, change in IgG level from baseline) that were significantly higher than their respective control values (329 and -32, Wilcoxon p value = 0.01). Receiver operator curves estimated a cutoff of 625 U as the optimal sensitivity (0.86 [0.37, 1.0]) and specificity (0.86 [0.37, 1.0]) for predicting Cryptosporidium infection. These data suggest that the enzyme-linked immunosorbent assay technique can be an effective epidemiologic tool to monitor Cryptosporidium infection in immunocompromised populations.
Cryptosporidium oocysts are regularly detected in treated and untreated water and have been associated with both food- and waterborne outbreaks (1,2). Of particular concern are immunocompromised persons, among whom HIV-infected persons represent a large group at risk for cryptosporidiosis (3-8). Cryptosporidiosis in HIV-infected persons may be chronic and is associated with substantial mortality. Recent evidence suggests that, in addition to their protracted course of infection, HIV-infected persons may be at higher risk for acquiring infection (9). The introduction of highly active antiretroviral therapy has decreased the incidence of cryptosporidiosis among HIV-positive persons (10), but there are no data to suggest that the incidence of exposure has been reduced.
In part because of limited surveillance tools, much is still unknown about the natural history of cryptosporidiosis (11). To confirm Cryptosporidium infection, stool specimens are often examined by microscopy. For epidemiologic studies, this method is problematic because of the short duration of oocyst excretion, the poor sensitivity of the procedure (12), and the amount of laboratory personnel time needed. Moreover, many physicians are unaware of cryptosporidiosis (13). Therefore, since most laboratories examine stools specifically for Cryptosporidium only on physician request (14), cryptosporidiosis is generally underdiagnosed.
Serologic assays provide an alternative to parasitologic methods for monitoring Cryptosporidium infections. Although many previous studies have used crude extracts of disrupted oocysts as the antigen in an enzyme-linked immunosorbent assay (ELISA), assays based on detection of antibody responses to specific Cryptosporidium antigens by immunoblot are more sensitive and specific. When the immunoblot is used, persons exposed to Cryptosporidium in outbreak settings have characteristic responses to 27-kDa (immunoglobulin [Ig] G) and 17-kDa (IgA, IgG) antigens, which are found on the surface of sporozoites, the infective stage of the parasite. In studies of volunteers exposed to C. parvum, antibodies directed against these antigens were correlated with lower levels of oocyst excretion and with protection from symptomatic infection (15).
From previous studies measuring responses to crude oocyst antigen, it is clear that HIV-positive persons can make antibody responses to C. parvum (16); however, these responses have not been assessed by the newer assay formats. Consequently, little is known about the ability of HIV-positive persons to mount an immune response to defined C. parvum antigens. The question remains whether HIV-positive persons can mount an antibody response, and, if so, whether the magnitude of the response is associated with CD4 count. To address these issues, we examined 28 clinically confirmed cases of cryptosporidiosis, assaying IgG responses for the 11 cases in which we had blood samples collected after the date of diagnosis.
Study Population
The San Francisco Men's Health Study (SFMHS) was a prospective study of the epidemiology and natural history of AIDS in a cohort of 1,034 single men between the age of 25 and 54 years (17). The subjects were recruited by multistage probability sampling and followed from 1984 through 1992. The men were followed every 6 months with an interview, a complete physical examination, and collection of clinical specimens. Serum banks have been maintained in liquid nitrogen since the beginning of the study. The institutional review boards of the University of California, Berkeley, and the Centers for Disease Control and Prevention approved this project.
A person was reported to have had cryptosporidiosis if he answered yes to the following question: "Since we last interviewed you (6 months ago), did a doctor or other medical practitioner tell you that you had cryptosporidiosis?" All diagnoses were based on finding oocysts in stool samples. Although we have no information on the illness status of these cases, it is unlikely that stool samples would have been collected for asymptomatic patients.
Records from the SFMHS were reviewed to identify persons diagnosed with C. parvum infection. Of 28, 11 had at least one serum sample collected after the date of diagnosis of cryptosporidiosis. To analyze antibody decay, we excluded persons with chronic cryptosporidiosis infection by excluding those with a CD4 count <200, on the assumption that these persons were at high risk for chronic cryptosporidiosis infection.
A control was defined as a person who 1) never had a clinical diagnosis of cryptosporidiosis while under observation; 2) had a serum sample available within 3 months (based on the date of the blood sample used to measure the IgG response after diagnosis of cryptosporidiosis for a matched case); and 3) had a CD4 count at this blood sample date that was within 50 cells/µL of the case CD4 count. For each case, two controls were randomly selected from all possible controls who met these three criteria.
Rationale for IgG Analysis
Assays based on the detection of IgA, to date, lack sensitivity. IgM results with crude-antigen ELISAs and with immunoblot have suggested that IgM is directed primarily at carbohydrate epitopes. IgM assays thus tend to be characterized by low signal-to-noise ratios and poor specificity. In addition, IgM responses tend to be short-lived, exacerbating issues related to the sensitivity of detection of antibody responses.
ELISA
Antibody assays used either a recombinant Cp23 protein or a partially purified native antigen fraction isolated from oocysts by Triton X-114 detergent extraction and were performed as described (18). Briefly, antigens were diluted in 0.1 M Na HCO3 buffer at pH 9.6 to concentrations of 0.2 µg/mL (recombinant Cp23) or 0.28 µg/mL (Triton X-114-extracted antigen) and were used to sensitize 96-well plates overnight at 4°C (50 L/well; Immunlon 2, Dynatech Industries, McLean, VA). Plates were blocked with phosphate-buffered saline (PBS) (0.85% NaCl and 10 mM Na2PO4 at pH 7.2) containing 0.3% Tween 20 for 1 hour at 4°C, then washed four times with 0.05% Tween 20/PBS. Unknown sera were diluted 1:50 in 0.05% Tween 20/PBS and loaded in duplicate (50 µL/well). Four blank wells (buffer only), duplicate wells containing three positive control sera, and duplicate wells containing four negative sera were included on each plate. A twofold serial dilution (1:50 to 1:12,800) of a strong positive control was also included on each plate to generate a standard curve. The plates were incubated for 2 hours at room temperature. Bound antibodies were quantified by using a biotinylated mouse monoclonal antibody against human IgG (1:1,000 in 0.05% Tween 20/ PBS) (clone HP6017; Zymed Laboratories, South San Francisco, CA) and alkaline phosphatase-labeled streptavidin (1:500 in 0.05% Tween 20/PBS) (Life Technologies, Rockville, MD) with p-nitrophenylphosphate substrate (Sigma Chemical Co., St. Louis, MO) as described (18). Absorbances at 405 nm were measured with a Molecular Devices UVmax kinetic microplate reader (Sunnyvale, CA). Antibody levels of unknown samples were assigned a unit value based on the 9-point positive control standard curve with a four parameter curve fit. The 1:50 dilution of the positive control serum was arbitrarily assigned a value of 6,400 U. Arbitrary unit values were expressed per microliter of serum.
Statistical Analysis
IgG response measures were reported in arbitrary units based on the standard curve described above. These antibody responses were not normally distributed; consequently, the responses of the cases and controls were compared by using the Wilcoxon rank sum test. Least squares regression was used to examine the temporal degradation of antibody responses. A receiver operator curve (ROC) was constructed to examine the sensitivity and specificity of different cutoff values (joint confidence intervals were based on exact methods).
Table 1 summarizes antibody responses of cases and controls. When the CP23 antigen was used, the median IgG value of the sample collected after the diagnosis of cryptosporidiosis (1,334 U for the cases) was significantly different from the control samples collected at the same time (329 U) (p<0.05). The median net increase in IgG levels between the serum samples collected before and after the diagnosis date for cases (433) was also significantly different from that for controls (-32) (p<0.05). The time interval between date of diagnosis of cryptosporidiosis and date of the blood sample was 44 to 369 days. After diagnosis with cryptosporidiosis, the median IgG value, when TX17 antigen was used, was not significantly different for cases (140 U) and controls (56 U); however, the median increase in IgG levels between the blood samples before and after the diagnosis date for the cases (71 U) was significantly different from that for controls (-1 U) (p<0.05). IgG responses of cases and controls at enrollment into the SFMHS in 1984 were not significantly different, suggesting that in general IgG levels of cases were not distinguishable from those of controls when not associated with a cryptosporidiosis diagnosis. There were some notable exceptions, e.g., one control had IgG levels consistently above 1,000 in four measurements from 1984 to 1992. Detailed information on cases and controls is shown in Table 2.
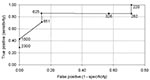
Next, we estimated the optimal cutoff or threshold value to be used as a predictor for whether an IgG response permitted classification of the subject as a case or a control. Based on the seven cases that had an IgG measurement within 200 days of the diagnosis date, Figure 1 shows an ROC curve (plot of false positives vs. true positives). A threshold of 625 U was estimated to maximize both the sensitivity (0.86 [0.37, 1.0]) and specificity (0.86 [0.37, 1.0]) of the data; this threshold was chosen as the value on the curve closest to the upper lefthand corner of the graph. We recalculated the ROC curve using the five cases that had an IgG measurement within 100 days of the diagnosis date, as well as the 10 cases with measurements within 300 days. Both analyses resulted in the same estimate for the optimal cutoff value of 625.
The kinetics of the antibody response to the CP23 antigen for each of the 11 cases are shown in Figure 2. Antibody responses were plotted relative to the cryptosporidiosis diagnosis for the 11 cases (time 0 represents the date of diagnosis). The plots were divided into three panels based on the 625-U threshold estimate. The top panel contains the five cases that had an IgG level <625 before and >625 after clinical diagnosis (Cases 1,2,3,4, and 7). All these cases had 3- to 20-fold increases in IgG levels. The middle panel contains the responses that had values >625 before and after clinical diagnosis (Cases 5,6,8, and 9). More detailed observations of these four cases revealed 1) data for Cases 5 and 9 were insufficient to assess the antibody response to infection since the last serum samples collected before diagnosis date were 3 and 1 years before infection, respectively; 2) the lack of response from Case 6 may be because the first available sample after diagnosis was obtained 1 year after diagnosis; and 3) a twofold increase in antibody occurred for Case 8 before the clinical diagnosis date. The bottom panel presents the two cases that remained below 625 U. Case 11 had no increase in IgG levels, and Case 10 had a twofold increase but remained below the threshold.
To determine the duration of antibody response, we analyzed the relationship between antibody level and the time interval between infection and sample collection. To reduce the likelihood that chronic infections would interfere with the analysis, we restricted our analysis to persons with CD4 counts >200. Although sample numbers were small (n = 6), this preliminary analysis suggests that CP23 responses decline to baseline approximately 300 days after an initial response of 3,200 U. Further studies will be necessary to confirm this conclusion.
Study of the natural history of cryptosporidiosis has been limited because of the difficulty of collecting data during the acute phase of the disease. Often, incidence rates are too low to make prospective studies feasible. The blood bank from the SFMHS provided a unique opportunity to study the serologic responsiveness of a cohort of HIV-positive homosexual men clinically confirmed with cryptosporidiosis. Two features of this database make it well suited for a serologic study of cryptosporidiosis: 1) blood was sampled regularly at 6-month intervals from 1984 to 1994, and yearly from 1994 to 1997; and 2) reporting of cryptosporidiosis increased because it is an AIDS-defining condition. We identified 28 clinically diagnosed cases; however, only 11 cases had blood samples both before and after the diagnosis date. To a large extent this was because many of the patients died from Cryptosporidium infection. Even when restricted to the 11 cases of cryptosporidiosis with specimens available after diagnosis, the analysis clearly demonstrated the ability of the ELISA using CP23 antigen to discriminate cases from matched controls. These results suggest that the ELISA is a viable approach to identifying recently infected HIV-positive persons. If augmented with data on incidence of diarrhea, this approach could be used to provide valuable estimates of the level of asymptomatic Cryptosporidium infection.
One concern with a seroprevalence study in an HIV-positive cohort such as homosexual men in San Francisco is that Cryptosporidium exposure might be ubiquitous and chronic. Most of the controls, however, had IgG levels that were significantly below case levels, suggesting that antibody levels are not continuously high in HIV-positive persons. In addition, the IgG responses of cases and controls at enrollment into the SFMHS in 1984 were not significantly different, suggesting that cases were not inherently more responsive to C. parvum than controls. The fact that the IgG response of the cases after infection differed from that of controls suggests that this cohort either had a low frequency of exposure or a relatively rapid decay of the antibody response. Our preliminary analysis suggests that a response would decay to control levels after approximately 1 year. This result is consistent with those of other studies (19).
Two limitations to this population-level estimate of IgG degradation are 1) our sample size was small, and 2) chronic infection and multiple exposures may interfere with the natural decay of the IgG response. Because of this small sample size, we were not able to address some potentially interesting and relevant issues, such as the relationship between serologic response and CD4 count. Since this study provides us with criteria for Cryptosporidium exposure, our future studies will not be limited to clinically confirmed cases and will therefore be able to obtain greater sample sizes.
With regard to the second limitation, we assumed that chronic infection was a potential problem and therefore excluded from the analyses subjects with a CD4 count <200/µL--that is, low CD4 count was used as a surrogate indicator for high risk for chronic infection (9,20). Although this was an indirect method of removing chronically infected cases, it provided us with a result that was consistent with previous studies of the kinetics of the antibody response to other antigens. Specifically, when an infection is not chronic, antibody levels decay over time.
The results of our study must also be interpreted in light of the fact that there was no information on the magnitude and timing of the exposure to C. parvum nor on prior exposures to C. parvum for these cases. These complications may explain why we have three patterns of responses (Figure 2): 1) The strong responders (Cases 1,2,3,4,7), who had low initial levels of IgG and who presumably had no or limited prior exposure; 2) The intermediate responders (Cases 5,6,8,9),who had initial antibody levels consistent with prior exposure or possible chronic infection. Three of these cases (5,8, and 9) had CD4 counts <100, suggesting that the presence of a chronic infection was possible; and 3) The nonresponders (Cases 10 and 11), who never produced levels above control values. The reason for this last pattern of response is not clear. Knowledge of exposure dose may help explain some of these differences. There is no evidence from these data that the level of CD4 count explained the magnitude of the humoral response. For example, Case 3, with a CD4 count of 96, had the strongest IgG response; Case 1, with a CD4 count of 500, had a relatively weak response. Factors responsible for determining the magnitude of the antibody response in infected persons have not been defined.
The Triton antigen (TX17) in this study was less useful than the CP23 antigen in distinguishing cases from controls. Although the TX17 could make this distinction based on the net antibody response to infection, these responses were relatively low. Assays based on use of the TX17 antigen have performed well in previous studies of outbreak populations. A possible reason for our results could be that the antibody response to this antigen is shorter lived. Alternatively, immunodeficient persons may not respond fully to this antigen. Neither explanation is well supported by the data. Cases 5 and 9 had low responses, even though blood samples were collected within 47 days of diagnosis, at a time when peak responses would be expected. Likewise, the second explanation is not supported by the data since one of the three strong responders was Case 3, who had a CD4 count of 96.
Results from this serologic study suggest that surveillance activities could be designed using a serologic test based on the CP23 antigen to estimate the number of recent infections of Cryptosporidium. Two pieces of information required before this test can be used are the definition of the optimal threshold IgG value that would define an infection event and the definition of a recent infection. ROC (Figure 1) provides both a method to optimize the choice of a threshold value to identify a case, based on the desired specificity and sensitivity, and a definition of recent infection, based on the decay of the antibody response. ROC analyses suggest that a level >625 U indicates an infection occurred within the past 100 to 300 days.
These results suggest that CP23 has important utility in the study of the epidemiology and natural history of cryptosporidiosis in HIV-infected populations. The value of CP23 in studying other potentially immunocompromised populations (such as oncology patients, children, and the elderly) deserves investigation.
Dr. Eisenberg is an adjunct assistant professor at the School of Public Health, University of California, Berkeley. His area of research is environmental epidemiology with a particular focus on waterborne infectious diseases.
Acknowledgment
This project was funded by the Centers for Disease Control and Prevention's Emerging Infections Program (U50/CCU9155546-03).
References
- Guerrant RL. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–7. DOIPubMedGoogle Scholar
- Juranek DD. Cryptosporidiosis: sources of infection and guidelines for prevention. Clin Infect Dis. 1995;21(Suppl 1):S57–61.PubMedGoogle Scholar
- Manabe YC, Clark DP, Moore RD, Lumadue JA, Dahlman HR, Belitsos PC, Cryptosporidiosis in patients with AIDS: correlates of disease and survival. Clin Infect Dis. 1998;27:536–42. DOIPubMedGoogle Scholar
- Matos O, Tomás A, Aguiar P, Casemore D, Antunes F. Prevalence of cryptosporidiosis in AIDS patients with diarrhoea in Santa Maria Hospital, Lisbon. Folia Parasitol (Praha). 1998;45:163–6.PubMedGoogle Scholar
- Pedersen C, Danner S, Lazzarin A, Glauser MP, Weber R, Katlama C, Epidemiology of cryptosporidiosis among European AIDS patients. Genitourin Med. 1996;72:128–31.PubMedGoogle Scholar
- Sorvillo F, Beall G, Turner PA, Beer VL, Kovacs AA, Kraus P, Seasonality and factors associated with cryptosporidiosis among individuals with HIV infection. Epidemiol Infect. 1998;121:197–204. DOIPubMedGoogle Scholar
- Hoxie NJ, Davis JP, Vergeront JM, Nashold RD, Blair KA. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am J Public Health. 1997;87:2032–5. DOIPubMedGoogle Scholar
- Colford JM Jr, Tager IB, Hirozawa AM, Lemp GF, Aragon T, Petersen C. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival [see comments]. Am J Epidemiol. 1996;144:807–16.PubMedGoogle Scholar
- Pozio E, Rezza G, Boschini A, Pezzotti P, Tamburrini A, Rossi P, Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J Infect Dis. 1997;176:969–75. DOIPubMedGoogle Scholar
- Kim LS, Hadley WK, Stansell J, Cello JP, Koch J. Declining prevalence of cryptosporidiosis in San Francisco. Clin Infect Dis. 1998;27:655–6. DOIPubMedGoogle Scholar
- Griffiths JK. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. DOIPubMedGoogle Scholar
- Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol. 1991;29:1323–7.PubMedGoogle Scholar
- Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? A survey of Connecticut physicians. Arch Intern Med. 1997;157:1017–22. DOIPubMedGoogle Scholar
- Roberts CL, Morin C, Addiss DG, Wahlquist SP, Mshar PA, Hadler JL. Factors influencing Cryptosporidium testing in Connecticut. J Clin Microbiol. 1996;34:2292–3.PubMedGoogle Scholar
- Moss DM, Chappell CL, Okhuysen PC, DuPont HL, Arrowood MJ, Hightower AW, The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis. 1998;178:827–33. DOIPubMedGoogle Scholar
- Ungar BL, Nash TE. Quantification of specific antibody response to Cryptosporidium antigens by laser densitometry. Infect Immun. 1986;53:124–8.PubMedGoogle Scholar
- Winkelstein W, Samuel M, Padian N, Wiley A, Lang W, Anderson RE, The San Francisco Men's Health Study: III. Reduction in human immunodeficiency virus transmission among homosexual/bisexual men, 1982-86. Am J Public Health. 1987;76:685–9. DOIPubMedGoogle Scholar
- Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–92.PubMedGoogle Scholar
- Moss DM, Bennett SN, Arrowood MJ, Wahlquist SP, Lammie PJ. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am J Trop Med Hyg. 1998;58:110–8.PubMedGoogle Scholar
- Flanigan T, Whalen C, Turner J. infection and CD4 count. Ann Intern Med. 1992;116:840–2.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 6—December 2001
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Joseph Eisenberg, 140 Warren Hall, MC 7360, School of Public Health, University of California, Berkeley, CA 94720-7360, USA; fax: 510-642-5815
Top