Volume 8, Number 7—July 2002
Research
Time-Space Clustering of Human Brucellosis, California, 1973–1992 1
Abstract
Infection with Brucella spp. continues to pose a human health risk in California despite great strides in eradicating the disease from domestic animals. Clustering of human cases in time and space has important public health implications for understanding risk factors and sources of infection. Temporal-spatial clustering of human brucellosis in California for the 20-year period 1973–1992 was evaluated by the Ederer-Myers-Mantel, Moran’s I, and population-adjusted Moran’s I procedures. Cases were clustered in concentrated agricultural regions in the first 5-year interval (1973–1977). Time-space clustering of human brucellosis cases in California late in the 20-year study period may reflect the distribution of Hispanic populations. Public health programs in California should focus on educating Hispanic populations about the risk of consuming dairy products, such as soft cheeses, made from unpasteurized milk.
Brucellosis is associated with chronic debilitating infections in humans and reproductive failure in domestic animals (1–3). Person-to-person transmission of brucellae is extremely rare (2,4), and human infection may be an accidental expression of a more widespread problem in animals (5). Brucella species considered important agents of human disease are B. abortus (primary reservoir in cattle), B. melitensis (sheep and goats), and B. suis (swine) (6). B. melitensis and B. suis are considered more pathogenic for humans than B. abortus (7). Dogs are reservoirs of B. canis; human infection has been documented to result in disease (3,8,9) but does not constitute an important public health concern in the United States (2,10).
Control and eradication of brucellosis in domestic animals have important public health implications. Test-and-slaughter programs, in conjunction with vaccination, are the major method of control (11). Whole herd depopulation (12) can also be used when other methods have reduced disease prevalence to low levels. Livestock populations can be screened for brucellosis by serologic testing of individual animals (12–16) or by testing pooled samples such as bulk milk (12,14). Several vaccines are available for reducing infection in animal populations (11), thereby reducing transmission potential to humans.
Persons infected with Brucella spp. usually have signs and symptoms consistent with an influenzalike or septicemic illness, often with insidious onset. The symptoms and clinical signs most commonly reported are fever, fatigue, malaise, chills, sweats, headaches, myalgia, arthralgia, and weight loss (8,10,17,18). Fewer than 10% of human cases of brucellosis may be recognized and reported (19), likely because of this misleading clinical picture (2,8). The acute form of human brucellosis is characterized by an undulating fever, in addition to the signs and symptoms mentioned. Lack of appropriate therapy during the acute phase may result in localization of bacteria in various tissues and lead to subacute or chronic disease that can have serious clinical manifestations (6,8,10).
Most cases involving field strains of Brucella can be traced to domestic food animals (5), and the prevalence of disease in livestock reservoirs reflects its occurrence in humans. Commonly, B. abortus and B. suis infections are associated with certain occupational groups, including farm workers, veterinarians, and meatpacking employees (6). Human B. melitensis infections occur more frequently in persons who do not have these occupational exposures (10). Persons usually become infected with brucellae through direct contact with infected animals or their products. Unpasteurized milk and processed dairy foods from infected animals are the major source of infection for the general population (7,10), and infected carcasses are the source of infection for workers in the meatpacking industry (20–22). Veterinarians can acquire brucellosis from assisting births in infected animals, as well as through inadvertent exposure to B. abortus strain 19 vaccine (5). Airborne transmission of bacteria to humans has also been documented in clinical laboratories and abattoirs (21, 23). Protective clothing and careful handling of infected animals can reduce occupation-related brucellosis (6,24), and avoiding unpasteurized dairy products should prevent infection in the general population (20).
The epidemiologic pattern of human infection with brucellae has been changing in the United States since 1947, when the number of reported cases was the highest ever recorded (6,321 total; 4.4 cases/100,000 population) (19,20). This change has been attributed to implementation of the state-federal Cooperative Brucellosis Eradication Program in 1934 and widespread milk pasteurization (19). B. abortus infection was common in the general population and before 1960 was the most frequent cause of human brucellosis (18). The relative importance of occupational exposures, especially in abattoir workers, steadily increased during the 1950s to 1970s (9,18,20,21). B. suis became the most frequent isolate in human cases from the mid-1960s to the early 1970s (9,18,19). The epidemiologic pattern of human brucellosis in the United States since the early 1970s may have shifted from an occupation-associated disease involving B. suis to one more common in the general population (23, 25). This change may be attributed to the swine brucellosis eradication program implemented in 1961 (22) and increased reporting of human B. melitensis infection (8,23,25), which was considered to have been eradicated from U.S. sheep and goats in 1972 (4). Hispanic populations of California are at increased risk for B. melitensis infection, with imported soft cheese the most commonly reported vehicle of exposure (23,25–27).
The objective of our study was to evaluate time-space clustering of reported human brucellosis cases in California for the 20-year period from 1973 to 1992. Determination of high-risk zones for human brucellosis may be useful in focusing education programs and public health funding.
Human brucellosis is a reportable disease in California; data from 1973 to 1992 were obtained through the Office of Statistics and Surveillance and the Veterinary Public Health Section, California Department of Health Services. Data included county of residence, year of diagnosis, and patients’ reported race and age. Data without personal identifiers were obtained for cases confirmed according to Centers for Disease Control and Prevention definitions (28). Basic descriptive data for reported cases of brucellosis from 1992 to 2001 were also obtained from the California Department of Health Services. California census information was downloaded from the Demographic Research Unit, California Department of Finance website, at http://www.dof.ca.gov/html/Demograp/druhpar.htm.
The 20-year study period was divided into four 5-year periods for calculating county-level human brucellosis incidence. The numerator for the incidence calculation was the total number of cases in residents of each county in the 5-year period. The denominator was the county population for the median year of the 5-year interval. Crude county-level incidences were calculated, as well as incidences adjusted for race and combined age and race. The data were adjusted for race by categorizing the total population into Hispanic and non-Hispanic segments and for age by grouping the population into <10-, 10–19-, 20–29-, 30–39-, 40–49-, 50–59-, and >60-year categories. Brucellosis incidence proportions were adjusted directly by using the population distribution of Sacramento County in 1990 as the standard. In brief, direct adjustment of proportions was done by first calculating the proportion of brucellosis in each specific age-race category for all counties. This proportion was then multiplied by the number of persons in the corresponding age-race category of the standard population (Sacramento County, 1990) to yield the expected number of cases. This expected number was summed over all age-race categories for each county and then divided by the total standard population (total population of Sacramento County, 1990) to yield the adjusted proportion.
Because of its insidious onset, the actual date of onset of Brucella infection is often difficult to determine retrospectively. Therefore, the date of diagnosis for each case, rather than onset of symptoms, was used for calculating incidence. Cases were considered to have originated in the patient’s county of residence for all calculations, although the person may not have become infected in that county. Data were not available to evaluate the effect of these assumptions on incidence calculations. ArcView Geographic Information System, version 3, (Redlands, CA) was used to visually represent spatial distribution of brucellosis cases and incidence proportions in California counties.
The Ederer-Myers-Mantel (EMM) procedure (29) was used to examine time-space clustering of human brucellosis cases in each California county during the 20-year study period. This one-sided test for clustering was implemented in an Excel spreadsheet program (Microsoft Corp., Redmond, WA). Separate race-specific analyses were also performed for cases in Hispanic and non-Hispanic residents. The EMM test is sensitive to departures from a static population over time and is therefore not recommended for situations involving more than five periods, as the baseline population may change. To account for this limitation, the 20-year period was divided into four 5-year periods in which the base population should not change meaningfully. This division of the period results in analysis of 232 counties (58´4) over 5 years each, instead of 58 counties over 20 years. Cases in Los Angeles County during 1978–1982, for instance, will not be linked to cases in Los Angeles before or after this 5-year period. Different periods for the same county are treated as completely independent.
The Moran’s I test for spatial clustering (30) was performed to evaluate distribution of incidence proportions of human brucellosis in California counties during the study period. Data were analyzed by RAMAS Cast, version 2.0 (Applied Biomathematics, Sebauket, NY). This statistical procedure examines values in adjacent areas (counties), is two-sided, and calculates a standard normal z-score in which a positive test statistic indicates a tendency toward a clustered distribution and a negative statistic a tendency toward a uniform (dispersed) distribution.
The Moran’s I test was performed independently for the four 5-year cumulative incidences of brucellosis in each California county during the study period. This analysis was also performed on the average incidence for each county during the entire 20-year period. Crude proportions as well as race- and age/race-adjusted proportions were analyzed.
A modification of the Moran’s I technique, the population-adjusted Moran’s I (Ipop), which adjusts for the underlying population density in each area (31), was used to evaluate spatial clustering of reported human brucellosis cases in California. The procedure was performed by using RAMAS Cast (Applied Biomathematics). Similar to the unadjusted Moran’s I, this statistical method is two-sided and calculates a standard normal z-score; however, the Ipop test is based on the numerator (number of cases) separate from the denominator (population at risk). Therefore, Ipop cannot be used for adjusted proportions because the data needed are numerators and denominators, rather than proportions.
The Ipop analysis was done by using numerator and denominator information from all four 5-year cumulative incidences of brucellosis in each California county during the study period. The total incidence for each county during the entire 20-year period was analyzed similarly, with mean county populations as the denominator. Spatial clustering was evaluated for the total population, as well as for Hispanic and non-Hispanic population segments.
Similar Ipop analyses were performed for reported cases in Hispanic and non-Hispanic segments of the population specific for B. abortus and B. melitensis infection. The causative Brucella species was determined either through bacteriologic isolation or determination of reported animal contact. Bacterial isolation was not done for all reported cases, and often the Brucella species was not determined because of concern about exposure risks for laboratory personnel. Patients reporting cattle as the primary animal contact were classified as having disease due to B. abortus when the infecting species was not determined. Similarly, patients reporting contact with goats (or goat cheeses) were classified as having infection with B. melitensis. This classification was necessary because the Brucella species involved was not identified for many cases.
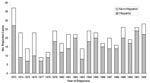
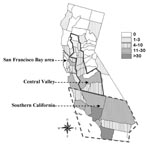
A total of 426 human cases of brucellosis were reported in California from 1973 to 1992. Ten cases were excluded from analysis because recorded permanent residence was not in California, leaving 416 for study. Except for the period 1974–1976, the number of Hispanic cases reported each year from 1973 to 1992 was greater than the total for all other ethnic groups combined (Figure 1). Hispanics accounted for 305 (73%) of the reported cases in this 20-year period. The number of reported cases was highest in southern California (66%), with another 14% from the Central Valley and 12% from the San Francisco Bay area (Figure 2).
The Brucella species was identified in 229 (55%) of the 416 cases analyzed. B. abortus was isolated from 39 cases, B. melitensis from 181, B. suis from 9. Expanding classification to include information about contact with animal species led to determination of 91 cases due to B. abortus and 200 cases due to B. melitensis.
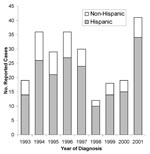
Human brucellosis cases since 1992, reported mainly from southern California counties, also affected mostly Hispanics (Figure 3). Hispanics accounted for 185 (77%) of the 240 total cases reported in California from 1993 to 2001, which was similar to data for 1973–1992. Southern California counties accounted for 136 (57%) of the total cases; another 22% were from the Central Valley and 14% from the San Francisco Bay area. Counties reporting the largest number of cases were Los Angeles (53 cases), San Diego (30 cases), and Orange (19 cases).
According to the EMM procedure, clustering was statistically significant for Hispanic, non-Hispanic, and total cases (Table 1); this procedure was also used to determine relative contribution to overall clustering by individual county (Table 2). Most of the clustering effect during 1973–1982 in Hispanic cases was found in southern and Central Valley counties. Clustering was most pronounced in Los Angeles County, with 23 cases reported in 1973 although the maximum number of cases expected from the EMM procedure was 10 (chi square 74.9, p<0.001). During 1988–1992, substantial clustering of Hispanic cases also occurred in the San Francisco Bay area and southern California. Non-Hispanic cases were significantly clustered in the Central Valley and San Francisco Bay area during 1983–1987. Cases also clustered in southern California during 1988–1992.
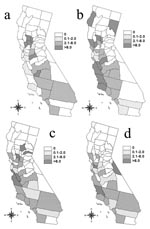
The Moran’s I procedure demonstrated significant spatial clustering of crude incidence of human brucellosis in California for the 5-year periods from 1973 to 1977 and 1983 to 1987 (Table 3). Spatial clustering remained statistically significant for 1973–1977 after incidences were adjusted for differences in age and race structure of the California counties, but significance during 1983–1987 was removed by this adjustment (Table 3). The largest adjusted incidences of human brucellosis occurred in the Central Valley, the most active agricultural region of California, during 1973–1977 (Figure 4). Spatial distribution of large adjusted incidences shifted away from highly agricultural zones in the later periods, and clustering was not statistically significant.
When the population as a whole was examined, the Ipop procedure showed substantial spatial clustering in all four 5-year study periods (Table 4). Significant clustering was also observed in the Hispanic-specific California population for all study periods except 1983–1987. Cases in the non-Hispanic population were not clustered during any period. The clustering effect in all tests resulted more from the number of cases within counties than from cases in adjacent counties (Table 4).
Significant spatial clustering was present in Hispanics infected with B. abortus during 1978–1982 (Table 5) and also in Hispanic populations infected with B. melitensis for all 5-year periods except 1983–1987. Cases of B. abortus or B. melitensis infection in non-Hispanic populations were not significantly clustered at any time during the 20-year study period.
The Hispanic segment of the California population has been shown to be at a higher risk for brucellosis during the period from the early 1970s to the early 1990s (25). The number of cases reported during 1973–1992 was higher in Hispanics than in all other ethnic groups combined. Spatial distribution of cases was centered in southern California and other areas of the state with large Hispanic populations.
Results of the EMM procedure showed significant time-space clustering of reported human brucellosis cases during the study period. Human brucellosis is not considered a contagious disease (2,19); therefore, clustering could result from common-source outbreaks or time-space clustering of factors that increase risk of infection. Human brucellosis is often associated with work-related (18,21,22) and foodborne (5,27) outbreaks, both of which were reported in California during 1973–1992 (25). The EMM procedure demonstrated clustering in total cases as well as Hispanic and non-Hispanic segments of the population but could not distinguish between risk-factor and common-source causes. Clustering in the Hispanic population was most evident in Los Angeles and other southern California counties during the study period. Non-Hispanic cases also tended to cluster in southern California, but not to the same degree as observed for Hispanics.
Spatial clustering of brucellosis incidence found by the Moran’s I technique during 1973–1977 was significant even after the data were adjusted for age and race structure of county populations. This finding suggests that, during this 5-year period, clustering of cases did not result simply from spatial distribution of Hispanic populations. Until recently, brucellosis had been reported to be most strongly associated with occupation; farm workers, veterinarians, and meatpacking employees were at highest risk. Counties with the highest adjusted incidences during this period were those with a high degree of agricultural activity, suggesting the importance of traditional exposures.
Crude incidences of county-level human brucellosis were significantly clustered for 1983–1987 based on Moran’s I. However, adjusting proportions for underlying race distributions removed the clustering, suggesting that clustering of human brucellosis cases during this time reflected the distribution of the Hispanic population. These findings confirm other reports that the epidemiology of human brucellosis is shifting from a disease of certain occupational groups to a foodborne disease of the general population, with Hispanics at greatest risk (23,25).
Reported cases of human brucellosis were significantly clustered in all four 5-year periods based on total population Ipop analysis. Reported cases in Hispanics were also significantly clustered in all 5-year periods except 1983–1988. Results also show that most clustering was due to the number of cases within counties, rather than in adjacent counties. This finding was most apparent in the Hispanic-specific analysis for 1988–1992. More than 100% of the clustering effect was estimated for cases in the same counties. Cases in adjacent counties during this period were dispersed, driving the test statistic in the other direction (negative clustering effect). Clustering in specific counties was strong enough to overcome this dispersion effect. Brucellosis incidences in non-Hispanics of California were not clustered at any time during the study period.
The Ipop analysis suggests that occurrence of human brucellosis in non-Hispanics of California is a random event that does not appear to cluster in certain counties or regions. In contrast, Hispanic cases showed a strong tendency to be clustered in certain counties. This tendency was especially true for 1988–1992, when nearly 50% (48/97) of Hispanic cases were reported in three nonadjacent California counties: Los Angeles (23 cases), San Diego (14 cases), and Alameda (11 cases).
Identification of spatial clustering of human disease for specific Brucella species can provide important epidemiologic information about animal reservoir and source of infection. Human infection due to B. abortus would be expected to cluster in counties with increased livestock activity early in the study period, when B. abortus was still endemic on some farms. Lack of significant clustering for 1973–1977 may have resulted from the small number of confirmed B. abortus infections and the resulting low statistical power for spatial tests. Spatial clustering of B. melitensis associated with Hispanic populations would be expected to be consistent throughout the 20-year study period. This observation held true except for 1983–1987, when no significant clustering was found for brucellosis cases in Hispanics or for B. melitensis-specific cases.
Data were analyzed by both the unadjusted and population-adjusted Moran’s I techniques, because of known characteristics of human brucellosis. The Ipop analysis is more powerful than the unadjusted Moran’s I (31), so the Ipop procedure is recommended when both numerator and denominator data are available. However, the null hypothesis of this statistical procedure is that cases are independent occurrences of disease in the underlying population at risk. Rejection of this null hypothesis leads to acceptance of the alternate hypothesis that clustering in disease occurrence is present. Human brucellosis is known to cluster in occupational and foodborne settings. Therefore, the statistical test may be biased because it is not able to subtract the effect of these outbreaks from the overall test statistic. This bias could be controlled if data were available for all outbreaks that occurred during the study period. The unadjusted Moran’s I technique has lower power and is therefore more conservative than the Ipop. The true nature of human brucellosis clustering in California during 1973–1992 most likely falls somewhere between the results of these two statistical analyses.
Human brucellosis continues to be a major public health concern in California even though the United States has effectively reduced the level of Brucella infection in domestic animals (32). Our results suggest that the epidemiology of risk factors for human infection due to Brucella spp. in California has changed. More traditional sources of infection are less important than the increased risk of Brucella spp. as foodborne pathogens. Traditional clustering of cases in concentrated agricultural regions was observed only for 1973–1977.
The Hispanic segment of the California population is at higher risk for disease due to Brucella infection than all other ethnic groups. Counties with high crude incidences of brucellosis correspond to those with large Hispanic populations. Increased risk has been attributed to certain dietary preferences, particularly for Mexican soft cheeses (23,25–27). Results suggest that time-space clustering of human brucellosis cases in California during the study period was predominantly due to clustered distribution of Hispanics in the state. However, Ipop and EMM results indicate residual temporal-spatial clustering of Hispanic cases in certain counties after the data were controlled for race through Hispanic-specific calculations. Counties with the highest adjusted incidences of human brucellosis during 1988–1992 were San Luis Obispo, Mono, and Alameda Counties. Only a single case was reported in Mono County during 1988–1992, but the small population size resulted in a relatively large incidence proportion.
Public health programs should focus on educating the Hispanic segment of the California population about the risks of consuming certain dairy products, such as soft cheeses, made from unpasteurized milk. Education must also extend to health-care providers who work in areas with large Hispanic populations likely to be exposed to illegally imported dairy products from areas where Brucella infection is still common in domestic animals. Efforts should be focused in southern California and San Francisco Bay area counties with the highest brucellosis incidences and absolute number of reported cases. More research focusing on the epidemiology of human brucellosis in California is necessary to aid in protection of its residents from disease.
Dr. Fosgate is a veterinarian and has received his PhD in epidemiology at the University of California, Davis. He has worked in private veterinary practice in the United States and has studied brucellosis in Nepal and Trinidad and Tobago. His research interests include diagnosis and control methods for Brucella abortus infection in species other than cattle.
Acknowledgment
We thank the many laboratory microbiologists who isolated Brucella strains at the California Department of Health Services Laboratory, Berkeley, as well as county epidemiologists and communicable disease officers throughout the state who completed case investigations. We also thank Joyanna Wendt for providing summary data for reported brucellosis cases after 1992.
References
- Corbel MJ. Microbiological aspects of brucellosis. Saudi Med J. 1993;14:489–502.
- Corbel MJ. Brucellosis: epidemiology and prevalence worldwide. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton (FL): CRC Press; 1989. p. 26–37.
- Nicoletti PL. Relationship between animal and human disease. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton (FL): CRC Press; 1989. p. 41–51.
- Hartigan P. Human brucellosis: epidemiology and clinical manifestations. Ir Vet J. 1997;50:179–80.
- Young EJ. Clinical manifestations of human brucellosis. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton (FL): CRC Press; 1989. p. 97–126.
- Nicoletti P. Control, eradication and prevention. In: Madkour MM, editor. Madkour’s brucellosis. New York: Springer; 2001. p. 280–5.
- Crawford RP, Huber JD, Adams BS. Epidemiology and surveillance. In: Nielsen KH, Duncan JR, editors. Animal brucellosis. Boca Raton (FL): CRC Press; 1990. p. 131–51.
- Huber JD, Nicoletti P. Comparison of the results of card, rivanol, complement-fixation, and milk ring tests with the isolation rate of Brucella abortus from cattle. Am J Vet Res. 1986;47:1529–31.PubMedGoogle Scholar
- MacMillan A. Conventional serologic tests. In: Nielsen KH, Duncan JR, editors. Animal brucellosis. Boca Raton (FL): CRC Press; 1990. p. 153–97.
- Wright PF, Nielsen KH, Kelly WA. Primary binding techniques for the serodiagnosis of bovine brucellosis: enzyme immunoassay. In: Nielsen KH, Duncan JR, editors. Animal brucellosis. Boca Raton (FL): CRC Press; 1990. p. 199–235.
- Nicoletti P. Brucellosis in animals. In: Madkour MM, editor. Madkour’s brucellosis. New York: Springer; 2001. p. 267–75.
- Shehabi A, Shakir K, El-Khateeb M, Qubain H, Fararjeh N, Shamat ARA. Diagnosis and treatment of 106 cases of human brucellosis. J Infect. 1990;20:5–10. DOIPubMedGoogle Scholar
- Buchanan TM, Faber LC, Feldman RA. Brucellosis in the United States, 1960–1972. An abattoir-associated disease. Part I. Clinical features and therapy. Medicine. 1974;53:403–13. DOIPubMedGoogle Scholar
- Wise RI. Brucellosis in the United States: past, present, and future. JAMA. 1980;244:2318–22. DOIPubMedGoogle Scholar
- Busch LA, Parker RL. Brucellosis in the United States. J Infect Dis. 1972;125:289–94.PubMedGoogle Scholar
- Buchanan TM, Hendricks SL, Patton CM, Feldman RA. Brucellosis in the United States, 1960–1972: an abattoir-associated disease. Part III. Epidemiology and evidence for acquired immunity. Medicine. 1974;53:427–39. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Brucellosis outbreak at a pork processing plant—North Carolina, 1992. MMWR Morb Mortal Wkly Rep. 1994;43:113–6.PubMedGoogle Scholar
- Taylor JP, Perdue JN. The changing epidemiology of human brucellosis in Texas, 1977–1986. Am J Epidemiol. 1989;130:160–5.PubMedGoogle Scholar
- Madkour MM. Epidemiologic aspects. In: Madkour MM, editor. Madkour’s brucellosis. New York: Springer; 2001. p. 21–32.
- Chomel BB, Debess EE, Mangiamele DM, Reilly KF, Farver TB, Sun RK, Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992—a shift toward foodborne transmission. J Infect Dis. 1994;170:1216–23.PubMedGoogle Scholar
- DeBess EE, Benjamin R. Foodborne brucellosis in an extended family, California, 1992. Berkeley: State of California Health and Welfare Agency, 1993; California Morbidity No. 3/4.
- Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep. 1997;46(RR-10):8–9.PubMedGoogle Scholar
- Ederer F, Myers MH, Mantel N. A statistical problem in space and time: do leukemia cases come in clusters? Biometrics. 1964;20:626–38. DOIGoogle Scholar
- Oden N. Adjusting Moran's I for population density. Stat Med. 1995;14:17–26. DOIPubMedGoogle Scholar
- Brucellosis declining, but still a problem in bison. J Am Vet Med Assoc. 1998;212:1684.
Figures
Tables
Cite This Article1 Preliminary results of this study were presented at the 9th Symposium of the International Society for Veterinary Epidemiology and Economics, August 6–11, 2000, Breckenridge, Colorado, USA.
Table of Contents – Volume 8, Number 7—July 2002
| EID Search Options |
|---|
|
|
|
|
|
|