Volume 14, Number 4—April 2008
Research
Rapid Typing of Transmissible Spongiform Encephalopathy Strains with Differential ELISA
Figure 1
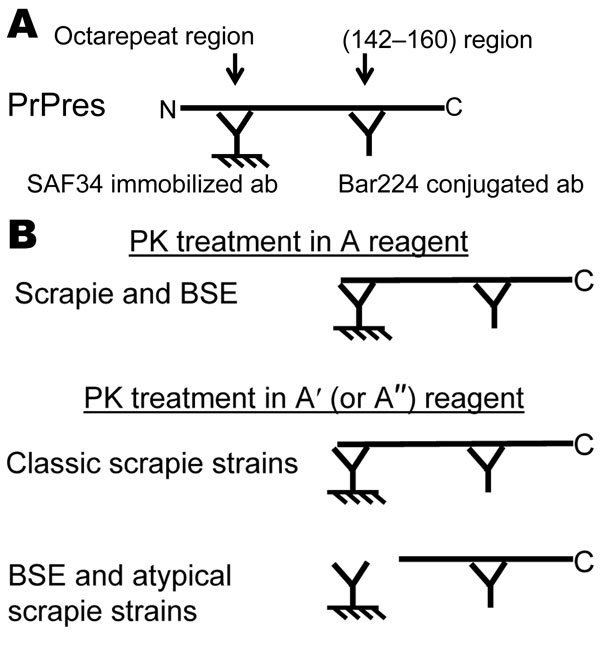
Figure 1. Principle of the 2-site immunometric typing assay. A) EIA screening test. In these conditions (Bio-Rad, Hercules, CA, USA), after mild proteinase K (PK) digestion, denatured PK-resistant prion protein (PrPres) is captured by the solid phase–immobilized antibody SAF34, recognizing the octarepeat region, and shown by the Bar224 tracer antibody, directed against the core of the protein. B) EIA typing test. In this test, the PrPres-containing sample is treated in 2 sets of PK conditions. In the first set of conditions (PK treatment in A reagent, Bio-Rad screening condition), the octarepeat region is maintained in scrapie- and bovine spongiform encephalopathy (BSE)–associated PrPres; in the second set of conditions (high PK concentration in A′reagent), BSE- and labile strain–associated PrPres is more sensitive to PK digestion than the classic PrPres associated with scrapie strains. Calculation of the ratio in the 2 conditions differentiates BSE and labile strains from classic scrapie strains. ab, antibody.