Volume 16, Number 2—February 2010
Research
Cost-effectiveness of Pharmaceutical-based Pandemic Influenza Mitigation Strategies1
Figure 2
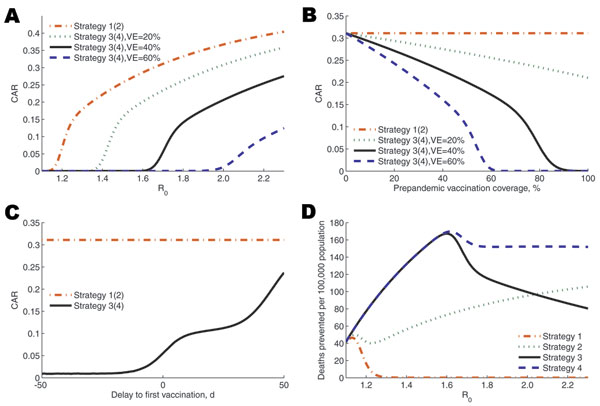
Figure 2. Sensitivity analyses of clinical outcomes as key parameters are varied. In A–C, the clinical attack rate (CAR) is displayed as a function of R0 and vaccine efficacy (VE) (A), vaccine coverage and VE (B), and the delay to vaccination (C). In D, deaths prevented per 100,000 population compared with no intervention is displayed as a function of R0.
1This material was compiled before the declaration of pandemic (H1N1) 2009 and concerns stockpiling for a future influenza pandemic.
Page created: December 10, 2010
Page updated: December 10, 2010
Page reviewed: December 10, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.