Volume 24, Number 1—January 2018
Research Letter
Visceral Leishmaniasis in Traveler to Guyana Caused by Leishmania siamensis, London, UK
Abstract
The parasite Leishmania siamensis is a zoonotic agent of leishmaniasis; infection in animals has been documented in Europe and the United States. Reported authochthonous human infections have been limited to Thailand. We report a case of human visceral Leishmania siamensis infection acquired in Guyana, suggesting colonization in South America.
A 65-year-old woman was admitted to a hospital in London, UK during March 2014 after collapsing in the street. She was anemic and mildly thrombocytopenic (hemoglobin level 8.1 g/dL, leukocyte count 4.62 × 109/L, platelet count 143 × 109/L). She had been unwell for 14 months, experiencing night sweats and a steady loss of energy but was otherwise asymptomatic. She reported no fever. On examination, she had hepatic enlargement and lymphadenopathy, was afebrile, and had normal liver function test results. She also had negative serologic test results for HIV, hepatitis B, hepatitis C, and the parasitic nematode spp. Strongyloides. Her CD4 count was 790 and rheumatoid factor was weakly positive; her immunoglobulin levels were within reference ranges.
The patient was from Guyana and migrated to the United Kingdom in 1967. Her relevant travel history comprised 2 recent visits to Guyana (Georgetown in 2012 and Freetown in 2013), Caribbean Grenada in 2012, Ghana in 2005, and France (Paris and Marseille) in 2003. We investigated for hematologic malignancy by bone marrow aspiration; Leishmania amastigotes were visible.
Results of serologic testing for Leishmania antibodies were negative by using a Rapydtest (rK39 RDT; Apacor Ltd., Berkshire, UK) and weakly positive by using the direct agglutination test (1:3,200; cutoff 1:1,600). Leishmania DNA was extracted from the sample. Leishmania–specific PCR amplification produced positive amplicons from kinetoplast minicircle, genomic heat shock protein 70, and internal transcribed spacer 1 (ITS1) targets. The kinetoplast–specific amplicon size appeared nearest to that of Leishmania major, which is not considered an agent of visceral leishmaniasis among humans.
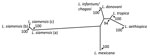
The size and restriction fragment length polymorphism banding pattern of the ITS1 amplicon was distinct from all previously sampled human Leishmania species. Sequencing of the ITS1 amplicon revealed either 99% or 100% identity to ITS1 sequences from Leishmania siamensis. Lower levels of identity were seen in homologs from other human Leishmania species. Phylogenetic analysis of these sequences against reference sequences from other Leishmania species confirmed they clustered with L. siamensis sequences as a monophyletic group, supported by bootstrap values of 100% (Figure). We saw a major divergence from other Leishmania sequences.
We treated the patient with liposomal amphotericin B (AmBisome [Gilead Sciences Ltd., London, UK) at a dose of 3 mg/kg given on days 1–5, and 7-day courses beginning on days 10 and 20. She responded well and her blood test results returned to reference values. She had some mild reversible renal impairment during treatment; she recovered and did not relapse during a 10-month follow-up period.
Autochthonous human visceral leishmaniasis caused by L. siamensis was thought to be geographically confined to Thailand (1–5). Many of those case-patients also showed evidence of immune deficiency, such as HIV infection (1,3). This patient had no evidence of immune deficiency, nor had one manifest during the follow-up period after her illness, yet she had a negative rK39 test result (which detects Leishmania antibodies) despite a visceral infection. Another patient with L. siamensis visceral leishmaniasis also had a negative rK39 test result (2); therefore, L. siamensis infections may not be detectable by rK39 testing.
The phlebotomine sand fly Sergentomyia (Neophlebotomus) gemmea is a possible vector for L. siamensis in Thailand (6–8). Sand flies from the Sergentomyia genus are generally zoophilic and therefore discounted as vectors of medically consequential Leishmania spp. Several Sergentomyia species are present in Europe and in South and North America, which may explain the presence of autochthonous zoonotic L. siamensis in these locations. Human infection with L. siamensis outside Thailand raises questions concerning transmission of this species to humans by anthropophilic phlebotomine sand flies or other species generally categorized as zoophilic.
Dr. Polley is a clinical scientist specializing in the diagnosis of human parasitic diseases through molecular methodologies. He is resident at the Hospital for Tropical Diseases where he is involved in developing new diagnostic technologies to detect Plasmodium, Leishmania, intestinal protozoa, and helminth infections.
Acknowledgment
S.D.P. is supported by the Foundation for Innovative New Diagnostics, through grants from the Federal Ministry of Education and Research of Germany, the KfW Entwicklungsbank, and the United Kingdom Department for International Development. P.L.C. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
References
- Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, et al. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012;86:821–4. DOIPubMedGoogle Scholar
- Osatakul S, Mungthin M, Siripattanapipong S, Hitakarun A, Kositnitikul R, Naaglor T, et al. Recurrences of visceral leishmaniasis caused by Leishmania siamensis after treatment with amphotericin B in a seronegative child. Am J Trop Med Hyg. 2014;90:40–2. DOIPubMedGoogle Scholar
- Noppakun N, Kraivichian K, Siriyasatien P. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg. 2014;91:869–70. DOIPubMedGoogle Scholar
- Leelayoova S, Siripattanapipong S, Hitakarun A, Kato H, Tan-ariya P, Siriyasatien P, et al. Multilocus characterization and phylogenetic analysis of Leishmania siamensis isolated from autochthonous visceral leishmaniasis cases, southern Thailand. BMC Microbiol. 2013;13:60. DOIPubMedGoogle Scholar
- Hitakarun A, Tan-ariya P, Siripattanapipong S, Mungthin M, Piyaraj P, Naaglor T, et al. Comparison of PCR methods for detection of Leishmania siamensis infection. Parasit Vectors. 2014;7:458. DOIPubMedGoogle Scholar
- Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, et al. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013;13:333. DOIPubMedGoogle Scholar
- Chusri S, Thammapalo S, Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health. 2014;45:13–9.PubMedGoogle Scholar
- Sukra K, Kanjanopas K, Amsakul S, Rittaton V, Mungthin M, Leelayoova S. A survey of sandflies in the affected areas of leishmaniasis, southern Thailand. Parasitol Res. 2013;112:297–302. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 24, Number 1—January 2018
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Spencer D. Polley, Department of Clinical Parasitology, 3rd Floor Mortimer Market Bldg, Capper St, Health Services Laboratories, London, UK
Top