
Figure 1. Procedure for selecting eligible references on the epidemiology of Haemophilus ducreyi as a causative agent of genital ulcers. GUDs, genital ulcer disease; STI, sexually transmitted infections.
Suggested citation for this article
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: December 17, 2015; Expiration date: December 17, 2016
Upon completion of this activity, participants will be able to:
• Distinguish the clinical presentation of genital ulcer disease with Haemophilus ducreyi
• Assess the means used to diagnose H. ducreyi infection
• Identify global areas disproportionately affected by H. ducreyi–related genital ulcer disease
• Assess worldwide trends in the epidemiology of infection with H. ducreyi
Thomas J. Gryczan, MS, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Thomas J. Gryczan, MS, has disclosed no relevant financial relationships.
Charles P. Vega, MD, Clinical Professor of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed the following financial relationships: served as an advisor or consultant for Lundbeck, Inc.; McNeil Pharmaceuticals; Takeda Pharmaceuticals North America, Inc.
Disclosures: Camila González-Beiras, BSc, MSc; Michael Marks, MBBS; Cheng-Yen Chen, PhD; Sally Roberts, MBChB, FRACP, FRCPA; and Oriol Mitjà, MD, PhD, have disclosed no relevant financial relationships.
The global epidemiology of Haemophilus ducreyi infections is poorly documented because of difficulties in confirming microbiological diagnoses. We evaluated published data on the proportion of genital and nongenital skin ulcers caused by H. ducreyi before and after introduction of syndromic management for genital ulcer disease (GUD). Before 2000, the proportion of GUD caused by H. ducreyi ranged from 0.0% to 69.0% (35 studies in 25 countries). After 2000, the proportion ranged from 0.0% to 15.0% (14 studies in 13 countries). In contrast, H. ducreyi has been recently identified as a causative agent of skin ulcers in children in the tropical regions; proportions ranged from 9.0% to 60.0% (6 studies in 4 countries). We conclude that, although there has been a sustained reduction in the proportion of GUD caused by H. ducreyi, this bacterium is increasingly recognized as a major cause of nongenital cutaneous ulcers.
Haemophilus ducreyi, a fastidious gram-negative bacterium, is the causative agent of chancroid, a genital ulcer disease (GUD). The organism is usually spread during sexual intercourse through microabrasions, and the disease usually manifests as multiple painful superficial ulcers associated with inguinal lymphadenitis (1). As a result of the painful nature of the lesions, patients usually seek immediate treatment, and asymptomatic carriage is therefore uncommon (2). In addition to causing GUD, H. ducreyi has been found in several recent studies to be a major cause of chronic skin ulceration in children from developing countries (3–6).
The global epidemiology of chancroid is poorly documented, and it is not included in World Health Organization estimates of the global incidence of curable sexually transmitted infections (STIs). There are some key challenges in interpreting data on the epidemiology of H. ducreyi as a causative agent of GUD. First, genital herpes cases are easily misdiagnosed as chancroid on clinical examination. Thus, reports based only on clinical diagnosis can be erroneous. Second, laboratory culture is technically difficult, and the highly sensitive and specific nucleic acid amplification tests, such as PCR, are rarely available outside national reference laboratories or specialized STI research settings, which makes it difficult to confirm clinical diagnoses.
Determination of the true global incidence of chancroid is made more difficult by widespread adoption of syndromic management for bacterial GUD (i.e., treatment with antimicrobial drugs effective against syphilis and chancroid) without microbiological confirmation in many countries. Therefore, countries often report only the total number of GUD cases. In addition, identification of GUD etiology is rarely conducted in resource-poor countries to validate syndromic management for which chancroid could also be common.
Earlier studies of tropical skin ulcers did not generally test for H. ducreyi, with the exception of a small number of case reports (7–11). There are major limitations in describing the prevalence of causative agents in tropical skin lesions that typically occur in children in rural areas where there is no access to laboratory facilities. Pathogens such as Fusobacterium fusiforme, Staphylococcus aureus, and Streptococcus pyogenes have been reported from Gram staining of exudative material collected from tropical ulcers (12). However, cultures or PCR testing for definitive identification of fastidious pathogens involved has not been traditionally conducted. The purpose of this study was to improve our understanding of the epidemiology of H. ducreyi infection through a systematic review of published data on the proportion of genital and skin ulcers caused by this bacterium.
A systematic review was conducted to identify all relevant studies that examined the etiology of GUD and nongenital skin ulcers involving H. ducreyi. We searched the National Library of Medicine through PubMed for “H. ducreyi,” “chancroid,” “genital ulcer,” OR “skin ulceration” AND “proportion” OR “prevalence.” The search was limited to studies published during January 1, 1980–December 31, 2014. In addition, we searched references of identified articles and other databases for other articles, and we reviewed abstracts, titles, and selected studies potentially containing information on chancroid epidemiology. We contacted researchers who were working with H. ducreyi to identify unpublished literature for inclusion. No language restrictions were set for searches.
The decision tree for inclusion or exclusion of articles is shown in Figure 1. We included studies if the proportion of etiologic agents in genital ulcers and nongenital skin ulcers, including H. ducreyi, was confirmed by laboratory techniques. Clinical diagnosis of chancroid is often based on the appearance of the ulcer, which is characteristically painful, purulent, and deep with ragged, undermined edges (Figure 2). However, because the appearance of these ulcers is similar to ulcers caused by other bacteria, clinical diagnosis can be nonspecific or insensitive and often requires laboratory confirmation (1). In addition, microscopy identification of typical morphologic features and serologic detection lack sensitivity and specificity (13,14). Thus, we only considered the following diagnostic methods as providing acceptable evidence of H. ducreyi infection: 1) isolation and identification by culture; or 2) PCR/real-time PCR.
For all qualifying studies, extracted data included study country, year of study, diagnostic test used for confirmation, total number of H. ducreyi–positive cases, and sample size. Descriptive analyses of extracted data were conducted, and the number of H. ducreyi–confirmed cases was divided by the total number of cases to calculate the proportion of cases caused by H. ducreyi. Studies qualifying for data extraction were grouped into 2 categories: studies conducted before 2000 and studies after 2000. This date separates studies before and after widespread implementation of syndromic management of GUD. Study sites were also plotted by geographic region. No quantitative metaanalysis was undertaken.
We identified 277 records in which we found 46 articles describing 49 studies on GUD that met our inclusion criteria (Tables 1, 2; Technical Appendix). All identified studies were based on cohorts of patients attending STI clinics, including 3 studies that enrolled only commercial sex workers. The age group for all cases was adults >18 years of age, except for 3 studies in Zambia, South Africa, and China, which included patients >16 years of age, and 1 study in Madagascar, which included patients >14 years of age. A total of 9 published studies and 2 unpublished reports that described nongenital skin ulcers caused by H. ducreyi were also included in our systematic review.
Laboratory confirmation of chancroid by PCR or culture was reported in 33 (67%) and 16 (32%) of the 49 studies, respectively. Of 16 studies that used culture, 7 (43%) used Mueller-Hinton agar with a nutritional supplement (e.g., IsoVitalex; Becton Dickinson, Franklin Lakes, NJ, USA), 1% used hemoglobin, and 5 (31%) used chocolate agar–based media; the remaining studies used other culture media. Five (31%) of 16 studies incubated agar plates at low temperatures (33°C–35°C), and 2 (12%) incubated plates at 36°C. Remaining articles did not specify incubating temperature.
Different PCR primer targets were used to amplify DNA sequences, including the 16S rRNA gene, the groEL gene, and the hemolysin gene. In addition to herpes simplex virus (HSV) PCR, 23 studies used a multiplex PCR that could simultaneously detect the 3 major causes of GUD (H. ducreyi, Treponema pallidum, and HSV types 1 and 2) (15). Studies encompassed 33 countries: 17 in Africa, 4 in Southeast Asia, 3 in Europe, 2 in the Middle East, 3 in South America, and 2 in the Caribbean, 1 in the United States, and 1 in Australia.
Of 49 studies on chancroid analyzed, 35 were published during 1980–1999 (Table 1) and 14 during 2000–2014 (Table 2). In general, data showed a clear decrease in the proportion of chancroid during 1980–2014 in all areas analyzed (Figure 3).
During 1980–1999, the proportion of genital ulcers caused by H. ducreyi in these studies ranged from 0.0% in Thailand and China to 68.9% in South Africa (Table 1). Eleven (31.4%) studies reported high proportions (>40%) of cases of infection with H. ducreyi. All of these studies were conducted in countries in Africa (Côte d’Ivoire, Gambia, Kenya, Lesotho, Senegal, South Africa, and Swaziland). Slightly lower proportions (20%–40% of cases) were observed in 15 (42%) studies: 10 in countries in Africa, 2 in the United States during localized outbreaks, 1 in Jamaica, 1 in the Dominican Republic, and 1 in India.
Only a few countries reported low proportions (<10%) of genital ulcers infected with H. ducreyi, including Singapore (8.3%), Peru (5%), Greece (4.6%), the Netherlands (0.9%), United States (3.1%), and Saudi Arabia (2.1%). The study in Saudi Arabia was conducted during 1995–1999; a total of 27,490 patients were examined for STIs. Chancroid was diagnosed by culture and was reported as the least common STI during this survey. The only studies that reported no cases of chancroid were conducted in Thailand in 1996 and China in 1999; both studies used multiplex PCR for detection of GUD cases.
During 2000–2014, the proportion of H. ducreyi infections was low (<10%) in all studies analyzed, except for 1 study in Malawi (15%) (Table 2). Studies in 5 countries (Kenya, Namibia, Zambia, Brazil, and Australia) did not report any cases of infection with H. ducreyi. Other studies reporting proportions of infections <10% were conducted in Botswana, Mozambique, South Africa, Uganda, Pakistan, and France. No reports were found for studies in North America, Southeast Asia, or the Caribbean.
During 1988–2010, several case reports described 4 children and 4 adults with nonsexually transmitted infections with H. ducreyi that manifested as lower leg lesions but no genital lesions. The reported case-patients were travelers who had been to Fiji (7), Samoa (8), Vanuatu (9), or Papua New Guinea (10) (Table 3). Outside the south Pacific region, a 5-year-old refugee from Sudan who had lower leg ulceration was also given a diagnosis of infection with H. ducreyi (11).
A cohort study conducted in Papua New Guinea in 2014 showed evidence that H. ducreyi is a major cause of chronic skin ulceration; H. ducreyi DNA was identified by PCR in 60.0% of skin lesions in children (3). Similar studies in other areas reported laboratory-confirmed skin ulcers in children caused by H. ducreyi in Papua New Guinea (6), Solomon Islands (4), Vanuatu (C.Y. Chen, pers. comm.), and Ghana (5) (Table 3).
Our review confirmed 2 major findings. First, reduction in the proportion of genital ulcers caused by H. ducreyi has been sustained for the past decade and a half. Second, there is increasing evidence that H. ducreyi is a common and newly recognized causative agent of chronic skin ulceration in children from developing countries.
In the 1990s, the global prevalence of chancroid was estimated to be 7 million (17). Chancroid was one of the most prevalent GUDs, particularly in resource-poor countries in Africa, Asia, Latin America, and the Caribbean (1; reference 51 in Technical Appendix). Recommendations to introduce syndromic management for treatment of GUD caused by bacteria were published by the World Health Organization in 1991 and fully implemented by 2000 (reference 61 in Technical Appendix). Since that time, global incidence of GUDs, particularly chancroid, has decreased substantially, and genital herpes viruses (HSV-1 and HSV-2) have become the predominant cause of GUD (reference 53 in Technical Appendix). Currently in Europe and the United States, chancroid is restricted to rare sporadic cases. Transmission of H. ducreyi remains ongoing in only a few countries that have limited access to health services (2,6).
Our data show marked decreases in the proportion of GUD caused by H. ducreyi in several countries. Spinola et al. reported similar conclusions obtained from 25 PCR-based studies (reference 62 in online Technical Appendix). For example, in Botswana (16), Kenya, (20), and South Africa (29), the proportion of GUD caused by H. ducreyi decreased from 25%–69% to negligible (0.0%–1.2%) levels (16; references 48,52 in Technical Appendix). Studies in Zambia (reference 56 in Technical Appendix), Namibia (reference 51 in Technical Appendix), and China (36) did not report any cases of chancroid during 2000–2009. A study in Thailand reported elimination of chancroid by introduction of a condom use program in the 1990s (reference 63 in Technical Appendix). Similar decreases have been reported from Cambodia and Sri Lanka, with rapid elimination of chancroid and congenital syphilis in most settings (reference 63 in Technical Appendix). However, these findings should be interpreted with caution because, given the short duration of infectivity, even a low prevalence of H. ducreyi in a population with GUD implies that a reservoir of infected persons with a high rate of sex partners is present.
Recent research has identified H. ducreyi as a previously unrecognized cause of nongenital skin ulcers in tropical areas. In 2013–2015, six studies in Papua New Guinea (3,6), the Solomon Islands (4), Vanuatu (C.Y. Chen et al., pers. comm.), and Ghana (5; C.Y. Chen et al., pers. comm.) showed that a high proportion of laboratory-confirmed skin ulcers were caused by H. ducreyi. Nearly half of the 690 enrolled patients with ulcers in these 6 studies had H. ducreyi detectable by PCR, whereas other bacteria, such as T. pallidum subsp. pertenue, the causative agent of yaws, were detected in 25% of patients.
These cases of infection with H. ducreyi confirmed by molecular analysis suggest that clinicians should be more aware of this newly recognized bacterium in skin ulcers of persons in tropical areas. In the context of new efforts to eradicate yaws, mass treatment with azithromycin in Papua New Guinea reduced the absolute prevalence of ulcers not caused by yaws, which were mainly caused by H. ducreyi, from 2.7% to 0.6% (prevalence ratio 0.23, 95% CI 0.18–0.29) at 12 months after treatment (6). However, persistence of H. ducreyi at low levels after mass treatment in Papua New Guinea (3) and Ghana (5) suggest that 1 round of mass treatment might not be successful in eradicating H. ducreyi skin ulcers.
Our review has several limitations. First, the increase in HSV-related GUD as a result of immunosuppression by HIV infection would result in a decrease in the proportion of chancroid among all GUD case-patients. Second, the lack of sequential studies performed in similar clinical settings at multiple time points precludes an optimal interpretation of the apparent decrease. Third, results might be affected by poor-quality data from many developing countries and might be inflated by publication bias. Fourth, PCR is more sensitive than culture. Therefore, increasing diagnostic yield might have partially masked the scale of the decrease in H. ducreyi as a cause of GUD.
In summary, we observed a quantitative and sustained reduction in cases of chancroid as a result of antimicrobial drug syndromic management and major social changes. In addition, data from several research groups indicate that H. ducreyi can cause nongenital skin lesions in persons residing in different regions. Further studies of this newly described pathogen skin disease association are required, and appropriate policies are needed that include the routine practice of managing tropical skin ulcers.
Ms. González-Beiras is a predoctoral fellow at Instituto de Higiene e Medicina Tropical, Lisbon, Portugal. Her primary research interests are strategies for elimination of neglected tropical diseases.
M.M. is supported by a Wellcome Trust Clinical Research Fellowship (WT102807).
Suggested citation for this article: González-Beiras C, Marks M, Chen CY, Roberts S, Mitjà O. Epidemiology of Haemophilus ducreyi infections. Emerg Infect Dis. 2016 Jan [date cited]. http://dx.doi.org/10.3201/eid2201.150425
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title: Epidemiology of Haemophilus ducreyi Infections
1. You are seeing a 21-year-old woman who recently emigrated from rural South Africa. She complains of genital ulcers. You consider whether Haemophilus ducreyi may be responsible for her symptoms. Which of the following statements regarding genital ulcer disease and H. ducreyi is most accurate?
A. H. ducreyi is a gram-positive organism
B. H. ducreyi promotes painless genital ulcers
C. Many patients with genital ulcer disease stemming from H. ducreyi have inguinal lymphadenitis
D. Most carriers of H. ducreyi are asymptomatic and are unaware of their infection
2. You perform an evaluation for genital ulcer disease in this patient. Which of the following statements regarding the diagnosis of H. ducreyi infection in the current study is most accurate?
A. Most cases of H. ducreyi were confirmed by PCR
B. All culture studies used low temperatures to grow H. ducreyi
C. All PCR studies used the hemolysin gene as a target
D. Multiplex studies that can detect H. ducreyi, Treponema pallidum, and herpes viruses were not used
3. Which of the following continents featured nations with the highest proportions of cases of H. ducreyi from 1980–1999?
A. South America
B. Australia and the South Pacific
C. Africa
D. Asia
4. Which of the following statements regarding trends in the diagnosis of chancroid in the current study is most accurate?
A. No study found a proportion of H. ducreyi genital ulcers above 10%
B. No African country reported rates of H. ducreyi below 10% between 2000 and 2014
C. More recent studies found that H. ducreyi was an emerging cause of chronic skin ulceration
D. The proportion of H. ducreyi found in genital ulcer disease remained relatively stable between 1980 and 2014
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |

Figure 1. Procedure for selecting eligible references on the epidemiology of Haemophilus ducreyi as a causative agent of genital ulcers. GUDs, genital ulcer disease; STI, sexually transmitted infections.
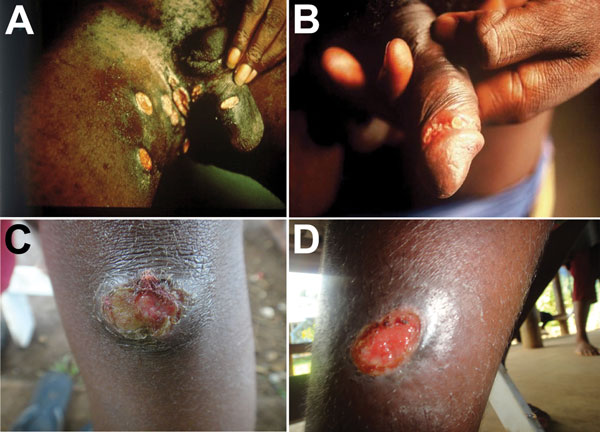
Figure 2. Ulcers caused by infection with Haemophilus ducreyi. A, B) Genital ulcers in adult patients from Ghana (provided by David Mabey). C, D) Skin ulcers in children from Papua New Guinea (provided by Oriol Mitjà).
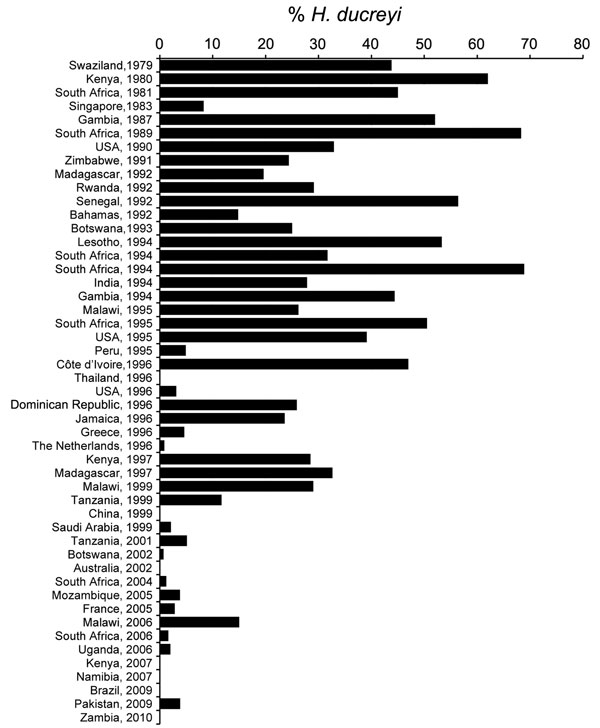
Figure 3. Trend of proportion of genital ulcers caused by infections with Haemophilus ducreyi, 1979–2010.
Characteristics of 35 studies of genital ulcers caused by Haemophilus ducreyi, 1980–1999*
| Area, reference† | Country | Year of study | Diagnostic method | No. patients with GUD | No. cases H. ducreyi infection | % (95% CI) |
|---|---|---|---|---|---|---|
| Africa | ||||||
| Paz-Bailey et al. (16) | Botswana | 1993 | Culture | 108 | 27 | 25.0 (17.7–33.9) |
| Steen (17) | Côte d’Ivoire | 1996 | PCR | NA | NA | 47 |
| Mabey et al. (18) | Gambia | 1987 | Culture | 104 | 54 | 51.9 (42.4–61.2) |
| Hawkes et al. (19) | Gambia | 1995 | M-PCR | 18 | 8 | 44.4 (24.5–66.2) |
| Nsanze et al. (20) | Kenya | 1980 | Culture | 97 | 60 | 61.8 (51.9–70.9) |
| Kaul et al. (21) | Kenya | 1997 | Culture | 189 | 54 | 28.5 (22.6–35.3) |
| Morse et al. (22) | Lesotho | 1994 | M-PCR | 105 | 55 | 53.3 (43.8–62.6) |
| Harms et al. (23) | Madagascar | 1992 | Culture | 12 | 61 | 19.6 (11.6–31.3) |
| Behets et al. (24) | Madagascar | 1997 | M-PCR | 196 | 64 | 32.6 (26.4–39.5) |
| Behets et al. (25) | Malawi | 1995 | M-PCR | 778 | 204 | 26.2 (23.2–29.4) |
| Hoyo et al. (26) | Malawi | 1999 | M-PCR | 137 | 41 | 29.0 (22.8–38.0) |
| Bogaerts et al. (27) | Rwanda | 1992 | Culture | 395 | 115 | 29.1 (24.8–33.7) |
| Totten et al. (28) | Senegal | 1992 | PCR | 39 | 22 | 56.4 (40.9–70.7) |
| Crewe-Brown et al. (29) | South Africa | 1981 | Culture | 100 | 45 | 45 (35.5–54.7) |
| Dangor et al. (30) | South Africa | 1989 | Culture | 240 | 164 | 68.3 (62.2–73.8) |
| Cheng et al. (31) | South Africa | 1994 | M-PCR | 538 | 171 | 31.7 (27.9–35.8) |
| Lai et al. (32) | South Africa | 1994 | M-PCR | 160 | 232 | 68.9 (62.7–74.5) |
| South Africa | 1998 | M-PCR | 94 | 186 | 50.5 (43.4–57.6) | |
| Meheus et al. (33) | Swaziland | 1979 | Culture | 155 | 68 | 43.8 (36.3–51.7) |
| Ahmed et al. (34) | Tanzania | 1999 | PCR | 102 | 12 | 11.7 (6.8–19.4) |
| Le Bacq et al. (35) |
Zimbabwe |
1991 |
Culture |
90 |
22 |
24.4 (16.7–34.2) |
| Asia | ||||||
| Wang et al. (36) | China | 1999 | M-PCR | 96 | 0 | 0.0 (0.0–3.8) |
| Risbud et al. (37) | India | 1994 | M-PCR | 302 | 84 | 27.8 (23.0–33.1) |
| Rajan et al. (38) | Singapore | 1983 | Culture | 670 | 56 | 8·3 (6·4–10·7) |
| Beyrer et al. (15) |
Thailand |
1996 |
M-PCR |
38 |
0 |
0.0 (0.0–9.1) |
| North America | ||||||
| Dillon et al. (39) | United States | 1990 | Culture | 82 | 27 | 32.9 (23.7–43.6) |
| Mertz et al. (40) | United States | 1995 | M-PCR | 143 | 56 | 39.1 (231.5–47.3) |
| Mertz et al. (41) |
United States |
1996 |
M-PCR |
516 |
16 |
3.1 (1.9–4.9) |
| South America | ||||||
| Sanchez et al. (42) |
Peru |
1995 |
M-PCR |
61 |
3 |
4.9 (1.6–13.4) |
| Caribbean | ||||||
| Sanchez et al. (42) | Dominican Republic | 1996 | M-PCR | 81 | 21 | 25.9 (17.6–36.4) |
| Behets et al. (43) | Jamaica | 1996 | M-PCR | 304 | 72 | 23·6 (19.2–28.7) |
| Bauwens et al. (44) |
Bahamas |
1992 |
PCR |
47 |
7 |
14·8 (7.4–27.6) |
| Middle East | ||||||
| Madani et al. (45) |
Saudi Arabia |
1999 |
Culture |
3,679 |
78 |
2.1 (1.7–2.5) |
| Europe | ||||||
| Kyriakis et al. (46) | Greece | 1996 | Culture | 695 | 32 | 4.6 (3.2–6.4) |
| Bruisten et al. (47) | The Netherlands | 1996 | M-PCR | 368 | 3 | 0.8 (0.2–2.3) |
*GUD, genital ulcer disease; NA, not available; M-PCR, multiplex PCR.
†References 41–47 provided in the online Technical Appendix (http://wwwnc.cdc.gov/EID/article/22/1/15-0425-Techapp1.pdf).
Characteristics of 14 studies of genital ulcers caused by Haemophilus ducreyi, 2000–2014*
| Area, reference† | Country | Year of study | Diagnostic method | No. patients with GUD | No. cases H. ducreyi infection | % (95% CI) |
|---|---|---|---|---|---|---|
| Africa | ||||||
| Paz-Bailey et al. (16) | Botswana | 2002 | PCR | 137 | 1 | 0.7 (0.1–4.0) |
| Mehta et al. (48) | Kenya | 2007 | M-PCR | 59 | 0 | 0.0 (0.0–6.1) |
| Phiri et al. (49) | Malawi | 2006 | M-PCR | 398 | 60 | 15.0 (11.8–18.9) |
| Zimba et al. (50) | Mozambique | 2005 | PCR | 79 | 3 | 3.8 (1.3–10.9) |
| Tobias et al. (51) | Namibia | 2007 | PCR | 199 | 0 | 0.0 (0.0–1.8) |
| O’Farrell et al. (52) | South Africa | 2004 | M-PCR | 162 | 2 | 1.2 (0.3–4.6) |
| Lewis et al. (53) | South Africa | 2006 | M-PCR | 613 | 10 | 1.6 (0.9–2.9) |
| Nilsen et al. (54) | Tanzania | 2001 | PCR | 232 | 12 | 5.1 (2.9–8.8) |
| Suntoke et al. (55) | Uganda | 2006 | M-PCR | 100 | 2 | 2.0 (0.5–7.0) |
| Makasa et al. (56) |
Zambia |
2010 |
PCR |
200 |
0 |
0 (0.0–1.8) |
| South America | ||||||
| Gomes Naveca et al. (57) |
Brazil |
2009 |
PCR |
434 |
0 |
0 (0.0–0.8) |
| Middle East | ||||||
| Maan et al. (58) |
Pakistan |
2009 |
Culture |
521 |
20 |
3.8 (2.5–5.8) |
| Europe | ||||||
| Hope-Rapp et al. (59) |
France |
2005 |
Culture |
278 |
8 |
2.8 (1.4–5.5) |
| Oceania | ||||||
| Mackay et al. (60) | Australia | 2002 | M-PCR | 64 | 0 | 0.0 (0.0–5.6) |
*GUD, genital ulcer disease; M-PCR, multiplex PCR.
†References 48–60 provided in the online Technical Appendix (http://wwwnc.cdc.gov/EID/article/22/1/15-0425-Techapp1.pdf).
Characteristics of 11 studies on skin ulcers caused by Haemophilus ducreyi, 1988–2014*
| Reference | Country | Year of study | Diagnostic method | No. patients with skin ulcers | No cases H. ducreyi infection | % (95% CI) |
|---|---|---|---|---|---|---|
| Marckmann et al. (7) | Fiji Islands | 1988 | Culture | 1 man | 1 | NA |
| Ussher et al. (8) | Samoa | 2005 | PCR | 3 girls <10 y of age | 3 | NA |
| McBride et al. (9) | Vanuatu | 2007 | PCR | 1 woman | 1 | NA |
| Peel et al. (10) | Vanuatu and Papua New Guinea | 2010 | PCR | 2 men | 2 | NA |
| Humphrey et al. (11) | Sudan | 2007 | PCR | 1 boy | 1 | NA |
| Mitjà et al. (3) | Papua New Guinea | 2013 | PCR | 90 | 54 | 60.0 (49.6–69.5) |
| Mitjà et al. (6) | Papua New Guinea | 2014 | PCR | 114 | 60 | 60.1 (54.3–65.5) |
| Marks et al. (4) | Solomon Islands | 2013 | PCR | 41 | 13 | 31.7 (19.5–46.9) |
| Chen et al.† | Vanuatu | 2013 | PCR | 176 | 68 | 38.6 (31.7–46.0) |
| Chen et al.† | Ghana | 2013 | PCR | 179 | 49 | 27.3 (21.3–34.3) |
| Ghinai et al. (5) | Ghana | 2014 | PCR | 90 | 8 | 8.8 (4.5–16.5) |
*NA, not applicable.
†Pers. comm.