Multidrug-Resistant Corynebacterium striatum Associated with Increased Use of Parenteral Antimicrobial Drugs
Volume 22, Number 11—November 2016
William O. Hahn, Brian J. Werth, Susan M. Butler-Wu
1, and Robert M. Rakita

Author affiliations: Author affiliation: University of Washington, Seattle, Washington, USA
Suggested citation for this article
Medscape CME Activity
This activity has been planned and implemented through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the American Nurses Credentialing Center (ANCC), the Accreditation Council for Pharmacy Education (ACPE), and the Accreditation Council for Continuing Medical Education (ACCME), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; and (4) view/print certificate.
Release date: October 12, 2016; Expiration date: October 12, 2017
Learning Objectives
Upon completion of this activity, participants will be able to:
• Analyze characteristics of infections with corynebacteria
• Distinguish anatomic sites of clinically relevant infection with Corynebacterium striatum
• Evaluate patterns of antimicrobial resistance of C. striatum
• Compare clinical outcomes of infections with C. striatum vs. coagulase-negative staphylococci
CME Editor
Thomas J. Gryczan, MS, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Thomas J. Gryczan, MS, has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Clinical Professor of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed the following financial relationships: served as an advisor or consultant for Allergan, Inc.; McNeil Consumer Healthcare; served as a speaker or a member of a speakers bureau for Shire Pharmaceuticals.
Authors
Disclosures: William O. Hahn, MD; Susan M. Butler-Wu, PhD; and Robert M. Rakita, MD, have disclosed no relevant financial relationships. Brian J. Werth, PharmD, has disclosed the following relevant financial relationships: received grants for clinical research from Merck, Allergan.
Abstract
Corynebacterium striatum is an emerging multidrug-resistant bacteria. We retrospectively identified 179 isolates in a clinical database. Clinical relevance, in vitro susceptibility, and length of parenteral antimicrobial drug use were obtained from patient records. For patients with hardware- or device-associated infections, those with C. striatum infections were matched with patients infected with coagulase-negative staphylococci for case–control analysis. A total of 87 (71%) of 121 isolates were resistant to all oral antimicrobial drugs tested, including penicillin, tetracycline, clindamycin, erythromycin, and ciprofloxacin. When isolated from hardware or devices, C. striatum was pathogenic in 38 (87%) of 44 cases. Patients with hardware-associated C. striatum infections received parenteral antimicrobial drugs longer than patients with hardware-associated coagulase-negative staphylococci infections (mean ± SD 69 ± 5 days vs. 25 ± 4 days; p<0.001). C. striatum commonly shows resistance to antimicrobial drugs with oral bioavailability and is associated with increased use of parenteral antimicrobial drugs.
Corynebacteria are a normal component of the microbiota of human skin and mucous membranes. At the University of Washington Medical Center (Seattle, WA, USA), Corynebacterium striatum has historically been the second most commonly isolated Corynebacterium species, after C. jeikium (1). Although C. striatum is a frequent colonizer (2), it might also be implicated as a true pathogen when isolated in multiple samples from sterile sites or from indwelling hardware and devices (2–9). Determination of whether an isolate represents infection, colonization, or contamination is based upon clinical judgment.
Although early reports indicated that C. striatum isolates were frequently susceptible to many antimicrobial drugs, including β-lactams, tetracycline, and fluoroquinolones (8–11), more recent studies have demonstrated increasing multidrug resistance (12–17). To clarify the spectrum of disease associated with C. striatum, we retrospectively extracted clinical information for immunocompetent patients with C. striatum isolates to determine clinical relevance, antimicrobial drug susceptibilities, and length of parenteral therapy. For patients with device-associated infections, we compared the length of parenteral therapy for C. striatum with that for coagulase-negative staphylococci, other low-virulence organisms that commonly colonize the skin.
Patients
We used an algorithm-based query of the De-Identified Clinical Data Repository maintained by the Institute for Translational Health Sciences of the University of Washington (Seattle, WA, USA) to identify 213 patients from the university medical center infected with C. striatum isolated from a clinical sample during 2005–2014. Adult patients (>18 years of age) were included if C. striatum was isolated from a specimen submitted for bacterial culture and identified to the species level. Because we were interested in whether C. striatum would be considered pathogenic in an immunocompetent population, patients with active immunosuppression were excluded from our analysis. We excluded 34 patients with immunosuppressant use at the time of culture with microbiological growth on the basis of pharmacy records (defined as documentation of use of corticosteroids, methotrexate, infliximab, adalimumab, tacrolimus, cyclosporine, mycophenolate mofetil, or rituximab), or with a diagnosis of active malignancy or HIV/AIDS according to codes from the International Classification of Diseases, 9th Revision. Our algorithm-based method was confirmed by using a manual chart review.
Bacterial Identification and In Vitro Drug Susceptibility Testing
Corynebacteria were identified to the species level if isolated in pure culture or if deemed to be clinically meaningful if present in a polymicrobial culture. Identification of corynebacteria was initially performed during 2005–2012 by using the RapID CB Plus Kit (Thermo Fisher Scientific, Waltham, MA, USA). This kit correctly identifies 95% of Corynebacterium isolates to the species level (18). During 2012–2014, corynebacteria were identified by using matrix- assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MALDI Biotyper and Biotyper software versions 3.0 and 3.1; Bruker Daltonics, Billerica, MA, USA) and a cutoff score of 2.0. Use of MALDI-TOF mass spectrometry with the Bruker system database correctly identifies corynebacteria to the genus level for >99% of isolates and correctly identified C. striatum in 100% (51/51) of clinical isolates tested (19).
Phenotypic susceptibility testing was performed by using the E-test (bioMérieux, Marcy l’Étoile, France) and Remel blood Mueller Hinton agar (Thermo Fisher Scientific) and incubation for 24–48 h at 35°C in ambient air, according to breakpoints of the Clinical and Laboratory Standards Institute (20). Because breakpoints have recently changed (21), we tested whether shifting the breakpoint would alter our results. Susceptibilities were available for penicillin, ciprofloxacin, clindamycin, erythromycin, and tetracycline. For patients from whom >1 isolate of C. striatum were obtained, we considered only the first isolate in our analysis.
Clinical Data Extraction
We obtained the following variables from chart review by manual extraction: patient location when the culture was obtained (i.e., inpatient versus outpatient), whether the culture grew C. striatum in pure culture or was polymicrobial, whether the treating physician considered the isolate to be clinically relevant or a contaminant, length of parenteral therapy administered, and whether adverse events were documented. If the treating physician did not comment on the isolate, the isolate was categorized as clinically irrelevant. Adverse events were defined as clinical event necessitating a change in antimicrobial agent and were graded according to the Common Terminology for Adverse Events (22). Serious adverse events were considered grade 3 or 4. The outpatient parenteral antimicrobial therapy practices at the University of Washington Medical Center monitor for adverse events by weekly measurement of a complete blood count and monitoring of renal function. We used these weekly measurements as a proxy to verify ongoing administration of outpatient parenteral therapy in combination with review of documentation in clinical notes.
Matched Case−Control Analysis
In a subset of patients with hardware-associated osteomyelitis or infections of implanted cardiac devices, we performed a matched case−control analysis to examine length of parenteral therapy in C. striatum cases compared with that for persons infected with coagulase-negative staphylococci (controls). Persons infected with coagulase-negative staphylococci were chosen as controls because isolates are frequently found in clinical samples (enabling appropriate matching), colonize the skin, and generally have low virulence, similar to C. striatum. Cases were matched to controls on the basis of age (± 5 years), site of infection, and presence or absence of hardware associated with the infection site.
Statistical Analysis
Statistical analysis was performed by using Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Data are reported as mean ± SE. Parametric data were compared by using the t-test, and nonparametric data were compared by using χ2 or Mann-Whitney U tests. A p value <0.05 was considered significant.
We identified 179 immunocompetent adult patients infected with C. striatum isolated from clinical specimens. A substantial proportion (48%, 86/179) of these isolates were believed to be from clinically relevant infections. For comparison, in our laboratory system during a similar period, ≈42,000 isolates of Staphylococcus aureus and 4,800 isolates of coagulase-negative staphylococci were recovered with in vitro susceptibilities determined.
Consistent with previous reports that C. striatum is a colonizer of the skin and mucous membranes, isolates were frequently reported in sputum and skin samples (65/179) (Figure 1). Interpretation of clinical relevance varied markedly by sample site. Isolates from deep surgical specimens, such as bone and surgical hardware, were generally considered to be clinically relevant (88%–95%), whereas isolates from urine, sputum, or skin were rarely considered to be clinically relevant (10%–15%) (Figure 1). Isolates from bronchoalveolar lavage samples were indicated by clinicians to be clinically relevant in 45% (9/20) of cases, and all of these patients were empirically treated with vancomycin for healthcare-associated pneumonia for a mean ± SE duration of 11 ± 1.9 days. No clinical failures for vancomycin therapy were documented.
Eighty percent (143/179) of isolates were found in an inpatient setting. Susceptibility testing was performed for 121/179 isolates, and 72% (87/121) were resistant to all oral antimicrobial drugs tested (Figure 2). The percentage of drug-resistant isolates obtained from the inpatient setting did not differ from that found in the outpatient setting (p = 0.27).
We determined in vitro susceptibilities for several antimicrobial drugs (Table). In general, MIC distributions were bimodal, whereby most drugs had low MICs or high MICs, except for vancomycin, which was universally active in vitro. The breakpoint of the Clinical and Laboratory Standards Institute for penicillin changed in 2015 (21) from 1 mg/L to 0.125 mg/L. This change increased the rate of isolates considered penicillin resistant from 85% to 98%, but did not affect the number of isolates without an oral drug option because all isolates with a penicillin MIC <1.0 mg/L were susceptible to tetracycline. Daptomycin was rarely formally tested in our laboratory during this period (n = 6), including the 2 patients we previously described (14). Initial MICs for daptomycin were 0.032–0.125 mg/L, but it was rarely used by clinicians in our cohort. Similarly, linezolid was rarely tested (n = 10), was uniformly active in vitro, but was not used clinically.
All patients with infections deemed to be clinically relevant were initially given vancomycin, except 1 patient with a documented allergy to vancomycin who received daptomycin. As expected, the length of time parenteral antimicrobial drugs were administered varied widely by anatomic site of infection and presence of a foreign body. Prosthetic joint infections were treated with parenteral therapy for 54 ± 7 days, and other hardware- or device-associated infections were treated for 65 ± 10 days. One patient with a ventricular assist device−associated infection who received vancomycin for 650 days was excluded from this analysis (outlier).
In a subset restricted to the 38 patients with hardware (including prosthetic joint) or device-associated infections deemed clinically meaningful, we successfully matched 27 patients with control patients infected with coagulase-negative staphylococci. Eleven patients could not be matched for site of infection or age. When we compared control patients infected with coagulase-negative staphylococci with patients infected with C. striatum, we found that those infected with C. striatum had a longer course of parenteral antimicrobial drugs (69 ± 5 days vs. 25 ± 4 days; p<0.001) (Figure 3). C. striatum isolates were more likely to be monomicrobial (14/27, 52%) than coagulase-negative staphylococci (5/27, 19%) (p = 0.02).
Five serious adverse events were associated with parenteral antimicrobial drugs in the C. striatum group and only 1 serious adverse event in the coagulase-negative staphylococci group (p = 0.19). The serious adverse events (all associated with vancomycin) were 1 drug reaction with eosinophilia and systemic symptoms syndrome, 2 acute kidney injuries with creatinine levels >3 times baseline values, and 3 absolute neutrophil counts <1,000 cells/mm3.
Because C. striatum can be a component of the skin microbiota, determination of whether microbiologic growth in a clinical sample represents an infection depends on clinical judgment. Previous investigations have described C. striatum primarily as a nosocomial pathogen, frequently in the setting of underlying malignancy or organ transplantation (15,23,24). Our report documents that clinicians encountering C. striatum in clinical samples of immunocompetent patients frequently consider the isolate to be a true pathogen.
Prior studies of small groups of patients have indicated that isolation of C. striatum from bone or a medical device is typically considered by the treating physician to be relevant (24–26), and our study confirms these findings. Furthermore, our study provides support for the pathogenic role of C. striatum in hardware-associated infections because we found that these infections with C. striatum were more likely to be monomicrobial than infections with coagulase-negative staphylococci. In contrast to some reports in which isolation from a respiratory sample was frequently determined to be clinically relevant (15,27,28), less than half of the respiratory isolates in our study were considered to reflect lower respiratory tract infections.
We documented that a multidrug-resistant phenotype of C. striatum directly affects clinical care. Osteomyelitis and hardware-associated infections are difficult to treat, often requiring a prolonged course of antimicrobial drugs. In some situations, guidelines recommend a limited duration of parenteral therapy followed by a longer period of oral therapy (29). A lack of well-tolerated oral treatment options active against C. striatum would be expected to lead to a longer duration of use of parenteral antimicrobial drugs for patients with these infections, and our matched case−control study confirmed this expectation.
The longer that parenteral antimicrobial therapy is necessary, the greater the likelihood of adverse events associated with intravenous access. These events include a rate of line events (mostly thrombosis) ranging from 5 to 17 episodes/100 devices and infection rates of 0.5 to 5 infections/100 lines (30). Although we documented more adverse events associated with treatment in the C. striatum group than in the coagulase-negative staphylococci group, this difference did not achieve a priori statistical significance, and we did not capture line thrombosis events. Furthermore, parenteral therapy is associated with substantially increased costs, even when comparing an inexpensive parenteral antimicrobial drug (vancomycin) with an expensive oral antimicrobial drug (linezolid) (31). In addition, parenteral options for C. striatum will be increasingly limited because our group and others have reported clinical failures caused by rapid development of high-level daptomycin resistance (14,16,25). Because daptomycin resistance can emerge rapidly, it is reasonable to assume that increased daptomycin use could also cause resistance to this drug.
One oral treatment option for multidrug-resistant C. striatum infections is linezolid. Although testing for C. striatum linezolid susceptibility is rarely performed in our clinical microbiology laboratory, to our knowledge, linezolid resistance has never been reported for corynebacteria. Nevertheless, linezolid is poorly tolerated during the long courses of treatment required for hardware- or device-associated infections and has shown a rate of adverse events leading to treatment discontinuation ranging from 34% to 80% (32,33). None of our patients in our study were treated with linezolid for a prolonged time, which most likely reflects reluctance of physicians to use an agent with such a high rate of toxicity.
We demonstrated that multidrug resistance was common even in isolates that were not considered to be clinically meaningful in an outpatient setting. We hypothesize that these resistant strains of C. striatum are probably circulating in the community rather than emerging under nosocomial pressure, but further studies would be needed to establish the ecologic niche of drug-resistant C. striatum strains.
Strengths of our study include a systems-wide approach to C. striatum infections in immunocompetent hosts and the large number of C. striatum case reports we reviewed. Detailed clinical information, including the treating physician’s interpretation of the sample, was linked to microbiological isolates. C. striatum will probably be recognized in more clinical settings because use of MALDI-TOF mass spectrometry enables C. striatum to be rapidly identified to the species level with a high degree of confidence without molecular techniques (19).
Limitations of our study include its retrospective nature and use of data from 1 health system, which restricted potential generalizability of the results. Given that our study was a retrospective study conducted over a 10-year period, we also cannot state whether the isolates are clonally related or reflect a diverse group with divergent mechanisms of drug resistance. In addition, we relied on the treating physician’s interpretation to determine the clinical relevance of an isolate. We have no other way of determining whether the isolate was causing disease, but we believe that this limitation is indicative of general clinical practice. Determining causality would require a different series of mechanistic investigations.
Our study demonstrates that C. striatum is an emerging multidrug-resistant pathogen. Our results highlight the need to identify corynebacteria to the species level, which is now readily performed by using MALDI-TOF mass spectrometry, and perform susceptibility testing for any isolate that is believed to be clinically meaningful. The frequent resistance of C. striatum to all easily tolerated oral antimicrobial drugs supports the need for development of new agents with good oral bioavailability and acceptable long-term safety profiles that are active against gram-positive organisms.
Dr. Hahn is an acting instructor in the Division of Allergy and Infectious Diseases at the University of Washington, Seattle, Washington. His primary research interest is host–pathogen interactions.
Acknowledgments
We thank Nic Dobbins, Eusung (Sally) Lee, and Robert Harrington for their assistance with the electronic database system (the Institute of Translational Health Science De-Identified Clinical Data Repository).
This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000423). W.O.H. was supported in part by an institutional training grant (T32 AI007044) from the National Institute of Allergy and Infectious Diseases. B.J.W. has received research funding from Merck (Kenilworth, NJ, USA) and Allergan (Parsippany–Troy Hills, NJ, USA).
References
Gavin SE,
Leonard RB,
Briselden AM,
Coyle MB.
Evaluation of the rapid CORYNE identification system for Corynebacterium species and other coryneforms. J Clin Microbiol.
1992;
30:
1692–
5.
PubMedGoogle Scholar Fernández Guerrero ML, Molins A, Rey M, Romero J, Gadea I. Multidrug-resistant Corynebacterium successfully treated with daptomycin. Int J Antimicrob Agents.
2012;
40:
373–
4 .
DOIGoogle Scholar Rufael DW,
Cohn SE.
Native valve endocarditis due to Corynebacterium striatum: case report and review. Clin Infect Dis.
1994;
19:
1054–
61.
DOIPubMedGoogle Scholar Mashavi M,
Soifer E,
Harpaz D,
Beigel Y.
First report of prosthetic mitral valve endocarditis due to Corynebacterium striatum: Successful medical treatment. Case report and literature review. J Infect.
2006;
52:
e139–
41.
DOIPubMedGoogle Scholar Marull J,
Casares PA.
Nosocomial valve endocarditis due to corynebacterium striatum: a case report. Cases J.
2008;
1:
388.
DOIPubMedGoogle Scholar Melero-Bascones M,
Muñoz P,
Rodríguez-Créixems M,
Bouza E.
Corynebacterium striatum: an undescribed agent of pacemaker-related endocarditis. Clin Infect Dis.
1996;
22:
576–
7.
DOIPubMedGoogle Scholar Tattevin P,
Crémieux AC,
Muller-Serieys C,
Carbon C.
Native valve endocarditis due to Corynebacterium striatum: first reported case of medical treatment alone. Clin Infect Dis.
1996;
23:
1330–
1.
DOIPubMedGoogle Scholar Martínez-Martínez L,
Suárez AI,
Rodríguez-Baño J,
Bernard K,
Muniáin MA.
Clinical significance of Corynebacterium striatum isolated from human samples. Clin Microbiol Infect.
1997;
3:
634–
9.
DOIPubMedGoogle Scholar Lagrou K,
Verhaegen J,
Janssens M,
Wauters G,
Verbist L.
Prospective study of catalase-positive coryneform organisms in clinical specimens: identification, clinical relevance, and antibiotic susceptibility. Diagn Microbiol Infect Dis.
1998;
30:
7–
15.
DOIPubMedGoogle Scholar Soriano F,
Zapardiel J,
Nieto E.
Antimicrobial susceptibilities of Corynebacterium species and other non-spore-forming gram-positive bacilli to 18 antimicrobial agents. Antimicrob Agents Chemother.
1995;
39:
208–
14.
DOIPubMedGoogle Scholar Martínez-Martínez L,
Pascual A,
Bernard K,
Suárez AI.
Antimicrobial susceptibility pattern of Corynebacterium striatum. Antimicrob Agents Chemother.
1996;
40:
2671–
2.
PubMedGoogle Scholar Yoo G,
Kim J,
Uh Y,
Lee HG,
Hwang GY,
Yoon KJ.
Multidrug-resistant Corynebacterium striatum bacteremia: first case in Korea. Ann Lab Med.
2015;
35:
472–
3.
DOIPubMedGoogle Scholar Campanile F,
Carretto E,
Barbarini D,
Grigis A,
Falcone M,
Goglio A,
Clonal multidrug-resistant Corynebacterium striatum strains, Italy. Emerg Infect Dis.
2009;
15:
75–
8.
DOIPubMedGoogle Scholar Werth BJ,
Hahn WO,
Butler-Wu SM,
Rakita RM.
Emergence of high-level daptomycin resistance in Corynebacterium striatum in two patients with left ventricular assist device infections. Microb Drug Resist.
2016;
22:
233–
7.
DOIPubMedGoogle Scholar Verroken A,
Bauraing C,
Deplano A,
Bogaerts P,
Huang D,
Wauters G,
Epidemiological investigation of a nosocomial outbreak of multidrug-resistant Corynebacterium striatum at one Belgian university hospital. Clin Microbiol Infect.
2014;
20:
44–
50.
DOIPubMedGoogle Scholar Tran TT,
Jaijakul S,
Lewis CT,
Diaz L,
Panesso D,
Kaplan HB,
Native valve endocarditis caused by Corynebacterium striatum with heterogeneous high-level daptomycin resistance: collateral damage from daptomycin therapy? Antimicrob Agents Chemother.
2012;
56:
3461–
4.
DOIPubMedGoogle Scholar Bernard K,
Pacheco AL.
In vitro activity of 22 antimicrobial agents against Corynebacterium and Microbacterium species referred to the Canadian National Microbiology Laboratory. Clin Microbiol Newsl.
2015;
37:
187–
98 .
DOIGoogle Scholar Hudspeth MK,
Hunt Gerardo S,
Citron DM,
Goldstein EJ.
Evaluation of the RapID CB Plus system for identification of Corynebacterium species and other gram-positive rods. J Clin Microbiol.
1998;
36:
543–
7.
PubMedGoogle Scholar Suwantarat N,
Weik C,
Romagnoli M,
Ellis BC,
Kwiatkowski N,
Carroll KC.
Practical utility and accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Corynebacterium species and other medically relevant Coryneform-like bacteria. Am J Clin Pathol.
2016;
145:
22–
8.
DOIPubMedGoogle Scholar Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; 2nd edition (M45–A2). Wayne (PA): The Institute; 2010.
Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; 3rd edition (M45–ED3). Wayne (PA): The Institute; 2015.
Otsuka Y,
Ohkusu K,
Kawamura Y,
Baba S,
Ezaki T,
Kimura S.
Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn Microbiol Infect Dis.
2006;
54:
109–
14.
DOIPubMedGoogle Scholar Watkins DA,
Chahine A,
Creger RJ,
Jacobs MR,
Lazarus HM.
Corynebacterium striatum: a diphtheroid with pathogenic potential. Clin Infect Dis.
1993;
17:
21–
5.
DOIPubMedGoogle Scholar McElvania TeKippe E,
Thomas BS,
Ewald GA,
Lawrence SJ,
Burnham C-AD.
Rapid emergence of daptomycin resistance in clinical isolates of Corynebacterium striatum… a cautionary tale. Eur J Clin Microbiol Infect Dis.
2014;
33:
2199–
205.
DOIPubMedGoogle Scholar Lee PP,
Ferguson DA Jr,
Sarubbi FA.
Corynebacterium striatum: an underappreciated community and nosocomial pathogen. J Infect.
2005;
50:
338–
43.
DOIPubMedGoogle Scholar Nhan T-X,
Parienti J-J,
Badiou G,
Leclercq R,
Cattoir V.
Microbiological investigation and clinical significance of Corynebacterium spp. in respiratory specimens. Diagn Microbiol Infect Dis.
2012;
74:
236–
41.
DOIPubMedGoogle Scholar Gomila M,
Renom F,
Gallegos MC,
Garau M,
Guerrero D,
Soriano JB,
Identification and diversity of multiresistant Corynebacterium striatum clinical isolates by MALDI-TOF mass spectrometry and by a multigene sequencing approach. BMC Microbiol.
2012;
12:
52.
DOIPubMedGoogle Scholar Osmon DR,
Berbari EF,
Berendt AR,
Lew D,
Zimmerli W,
Steckelberg JM,
;
Infectious Diseases Society of America.
Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis.
2013;
56:
e1–
25.
DOIPubMedGoogle Scholar Barr DA,
Semple L,
Seaton RA.
Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis.
2012;
31:
2611–
9.
DOIPubMedGoogle Scholar Stephens JM,
Gao X,
Patel DA,
Verheggen BG,
Shelbaya A,
Haider S.
Economic burden of inpatient and outpatient antibiotic treatment for methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res.
2013;
5:
447–
57.
PubMedGoogle Scholar Morata L,
Tornero E,
Martínez-Pastor JC,
García-Ramiro S,
Mensa J,
Soriano A.
Clinical experience with linezolid for the treatment of orthopaedic implant infections. J Antimicrob Chemother.
2014;
69(
Suppl 1):
i47–
52.
DOIPubMedGoogle Scholar Tang S,
Yao L,
Hao X,
Zhang X,
Liu G,
Liu X,
Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J.
2015;
45:
161–
70.
DOIPubMedGoogle Scholar
Suggested citation for this article: Hahn WO, Werth BJ, Butler-Wu SM, Rakita RM. Multidrug-resistant Corynebacterium striatum associated with increased use of parenteral antimicrobial drugs. Emerg Infect Dis. 2016 Nov [date cited]. http://dx.doi.org/10.3201/eid2211.160141
10.3201/eid2211.160141
Medscape CME Quiz
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Multidrug-Resistant Corynebacterium striatum Associated with Increased Use of Parenteral Antimicrobial Drugs
CME Questions
1. You are called to evaluate a female patient with a complicated medical history who is admitted to the hospital with fever and bacteremia. She has a history of diabetes mellitus and chronic kidney disease. She was treated with antibiotics for osteomyelitis of the left leg 1 year ago, and she underwent a right total knee arthroplasty last month. A preliminary result for her blood culture demonstrates a Corynebacterium species. What should you consider regarding infections with corynebacteria?
A. Corynebacteria are part of the normal skin flora
B. Corynebacterium striatum is the most commonly isolated corynebacteria in infections
C. C. striatum is invariably pathogenic in promoting infection
D. C. striatum has a long history of resistance to multiple antibiotics
2. You consider potential sources for this patient's infection. Which of the following infection sites with Corynebacterium striatum was most commonly judged as clinically meaningful in the current study?
A. Blood
B. Bone
C. Bronchoalveolar lavage
D. Surgical site
3. You need to recommend antibiotic therapy for this patient. What should you consider regarding resistance patterns of Corynebacterium striatum in the current study?
A. Approximately one-third of patients treated with vancomycin experienced clinical failure
B. Most isolates were from outpatient settings
C. Nearly three-quarters of isolates were resistant to all antibiotics tested
D. Rates of resistance were much higher among isolates from inpatient vs outpatient settings
4. Which of the following statements comparing patients with Corynebacterium striatum infections vs patients with coagulase-negative staphylococci in the current study is most accurate?
A. Patients with C. striatum infections received a longer average course of parenteral antibiotics
B. Patients with C. striatum infections were more likely to be treated with multiple antibiotics
C. Patients with C. striatum infections were less likely to experience complications related to infections
D. Patients with C. striatum experienced fewer complications related to the use of vancomycin specifically
Activity Evaluation
|
1. The activity supported the learning objectives.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
2. The material was organized clearly for learning to occur.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
3. The content learned from this activity will impact my practice.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
4. The activity was presented objectively and free of commercial bias.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
Figure 1
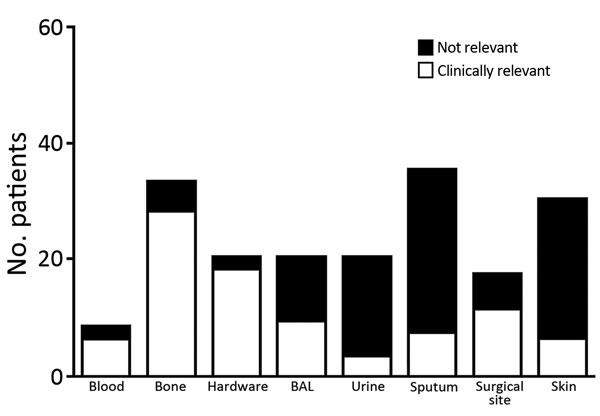
Figure 1. Patients infected with Corynebacterium striatum, by specimens isolated from a particular anatomic site, at the University of Washington Medical Center, Seattle, Washington, USA, 2005–2014. Hardware, surgical specimen obtained from a location anatomically in connection with a foreign device; BAL, bronchoalveolar lavage; urine, specimen isolated from a urine sample (we were unable to determine presence or absence of a catheter); surgical site, deep surgical specimen; skin, wound swab from a nonsurgical superficial specimen.
Figure 2
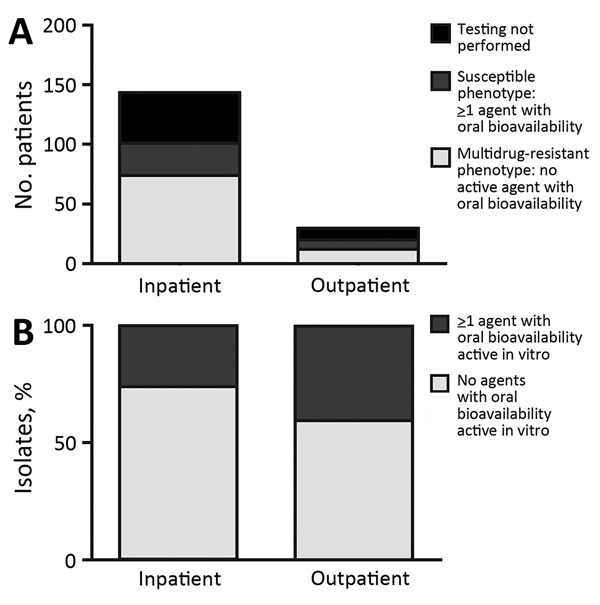
Figure 2. Numbers (A) and percentages (B) of Corynebacterium striatum isolates from patients at the University of Washington Medical Center, Seattle, Washington, USA, 2005–2014, with a multidrug-resistant phenotype for all antimicrobial drugs tested (penicillin, ciprofloxacin, clindamycin, erythromycin, and tetracycline). Inpatient or outpatient indicates clinical setting in which cultures were performed.
Figure 3

Figure 3. Length of use of parenteral intravenous antimicrobial drugs in matched case−control analysis of Corynebacterium striatum isolates and isolates of coagulase-negative staphylococci in patients with hardware-associated infections, University of Washington Medical Center, Seattle, Washington, USA, 2005–2014. Horizontal lines within boxes indicate median values, whiskers indicate minimum and maximum values, and boxes indicate 25th and 75th percentiles. Mean durations of parenteral antimicrobial drug use for patients infected with C. striatum and those infected with coagulase-negative staphylococci were compared by using the Mann-Whitney U test. CoNS, coagulase-negative staphylococci.
Table
Drug resistance patterns for Corynebacterium striatum isolates from patients at the University of Washington Medical Center, Seattle, Washington, USA, 2005–2014*
| Characteristic |
Penicillin, n = 121 |
Tetracycline, n = 119 |
Clindamycin, n = 103 |
Erythromycin, n = 72 |
Ciprofloxacin, n = 119 |
Vancomycin, n = 120 |
| Mean MIC |
18 |
34 |
209 |
66 |
51 |
0.6 |
| Median MIC |
8 |
32 |
256 |
16 |
32 |
0.5 |
| MIC range |
0.125–256 |
0.125–256 |
0.25–256 |
0.25–256 |
0.125–256 |
0.125–1 |
| MIC50 |
8 |
32 |
256 |
16 |
32 |
0.5 |
| MIC90 |
32 |
64 |
256 |
256 |
32 |
1 |


