Volume 10, Number 9—September 2004
Perspective
Potential Public Health Impact of New Tuberculosis Vaccines
Abstract
Developing effective tuberculosis (TB) vaccines is a high priority. We use mathematical models to predict the potential public health impact of new TB vaccines in high-incidence countries. We show that preexposure vaccines would be almost twice as effective as postexposure vaccines in reducing the number of new infections. Postexposure vaccines would initially have a substantially greater impact, compared to preexposure vaccines, on reducing the number of new cases of disease. However, the effectiveness of postexposure vaccines would diminish over time, whereas the effectiveness of preexposure vaccines would increase. Thus, after 20 to 30 years, post- or preexposure vaccination campaigns would be almost equally effective in terms of cumulative TB cases prevented. Even widely deployed and highly effective (50%–90% efficacy) pre- or postexposure vaccines would only be able to reduce the number of TB cases by one third. We discuss the health policy implications of our analyses.
Tuberculosis (TB) remains one of the leading causes of illness and death in the world. One third of the world’s population is estimated to be infected with Mycobacterium tuberculosis, the causative agent of TB (1). This reservoir of infected persons leads to ≈8 million new cases of TB and 2 million deaths each year. Approximately 80% of all new TB cases in the world occur in 22 countries that have incidence rates from 68 to 584 per 100,000 population (2). The priorities for TB control programs in these areas are identifying and treating active cases. Unfortunately, only 40% of smear-positive pulmonary cases are detected globally, and, of these cases, 28% to 80% are treated successfully (2). Most high-incidence countries also use the only available TB vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG). Although BCG is the most widely used vaccine in the world, its efficacy in preventing adult forms of TB is relatively poor, with an average efficacy ≈50% (3). A new, more effective vaccine would be expected to improve TB control substantially, and therefore, vaccine development is one of the highest priorities in TB research (4,5). The Gates Foundation recently provided nearly $83 million in grants to boost TB vaccine research (6).
Recent sequencing of the M. tuberculosis genome as well as new developments in proteomics and comparative genomics have led to renewed interest in developing new, more effective vaccines against TB (7,8). Vaccines currently under development include subunit vaccines (9), naked DNA vaccines (10,11), and attenuated mycobacteria, including recombinant BCGs expressing immunodominant antigens and cytokines (12). Phase I clinical trials of several of these vaccines are under way or scheduled to begin very soon (13,14). TB vaccines under development can be divided into two categories: preexposure or postexposure vaccines. Preexposure vaccines prevent infection and subsequent disease; these vaccines are given to uninfected persons. Postexposure vaccines aim to prevent or reduce progression to disease; these TB vaccines will be given to persons who are already infected with M. tuberculosis. In industrialized countries where TB incidence is low, a preexposure vaccine is the most effective for TB control (15). However, the most effective type of vaccine to control TB epidemics in high-incidence countries, where prevalence of latent TB infection is high, is not apparent. We use mathematical models to predict the potential public health effect of new TB vaccines for epidemic control in high-incidence countries. We evaluate the effect of both pre- and postexposure TB vaccines on two outcome variables: the number of new infections and the number of new cases of disease. We then discuss health policy implications of our analyses.
We used mathematical models to compare the potential public health impact of mass vaccination campaigns that used either pre- or postexposure vaccines. We assessed the public health impact in terms of the cumulative percentage of infections prevented and the cumulative percentage of TB cases prevented. We modeled the potential effect of vaccines in developing countries with a high incidence and prevalence of infection. Our simulated incidence ranged from 100 to 200 new TB cases per 100,000 persons per year, and we assumed that 28%–50% of the population was latently infected with M. tuberculosis. We also assumed that treatment rates were low to moderate (i.e., that 40%–60% of TB patients would be treated and cured). We modeled the potential public health impact of high-efficacy (50%–90%) vaccines and high vaccination coverage rates (60%–90%). We used two separate mathematical models to assess the effect of vaccination: a pre- and a postexposure vaccine model (see Appendix). Our models are similar to those developed by Lietman and Blower (15,16), but we extended them to include the possibility of reinfection of latently infected persons. We analyzed both of our models with uncertainty and sensitivity analysis based on Monte Carlo methods (17–20) (see Appendix for further details) to quantify the effect of vaccine efficacy, duration of vaccine-induced immunity, and vaccination coverage rates on the cumulative percentage of infections and TB cases prevented.
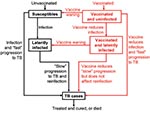
Both our vaccine models reflect the basic pathogenesis of TB (Figures 1 and 2), as in our previous models (21–27). When persons become infected with M. tuberculosis, one of the following can occur: 1) they can progress quickly to disease (with probability p); 2) they can become latently infected with M. tuberculosis (with probability 1 – p), and disease never develops; or 3) they can become latently infected with M. tuberculosis (with probability 1 – p) and slowly progress to disease (at rate v). Latently infected persons can also become reinfected (with a relative risk of θ) with a new strain of M. tuberculosis. We assessed the potential public health impact of 1,000 different postexposure and 1,000 different preexposure vaccines. Each vaccine had a different efficacy (from 50% to 90%) and average duration of vaccine-induced immunity (from 10 to 30 years). We modeled vaccination coverage rates from 60% to 90%. We modeled a mass vaccination campaign at year zero, and then continuous vaccination of each target population each subsequent year.
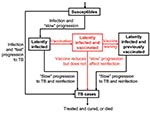
Our pre- and postexposure vaccine models were designed to vaccinate different populations: preexposure vaccines were designed for uninfected persons, and postexposure vaccines were designed for latently infected persons. We modeled vaccine efficacy for the 1,000 postexposure vaccines by the magnitude of the vaccine’s effect on reducing the rate of latently infected persons’ progressing to disease (Figure 1). Efficacy of preexposure vaccines is potentially more complex than that of postexposure vaccines, since preexposure vaccines have several potential mechanisms of action. Thus, we assumed that preexposure vaccines could act by three different mechanisms (Figure 2): 1) by reducing the risk for infection in the uninfected, 2) by allowing infection but reducing the probability of fast progression to disease, and 3) by allowing infection but reducing the rate of progression of latent infection to clinical disease. For each of our 1,000 preexposure vaccines, we varied these three potential mechanisms independently from 50% to 90%.
In terms of reducing the cumulative number of new infections with M. tuberculosis, we found that campaigns that used preexposure vaccines had substantially greater effectiveness than campaigns that used postexposure vaccines (Figure 3A). Preexposure vaccines quickly and substantially reduced the number of new infections; the median cumulative percentage of infections prevented (after 10 years of vaccination) was 46% (interquartile range [IQR] 40%–53%). The effectiveness of preexposure vaccines in preventing new infections diminished over several decades but remained fairly high. Postexposure vaccines had a considerably slower and smaller effect on reducing the number of new infections; the cumulative percentage of infections prevented rose from 0% (when mass vaccination began) and peaked after ≈10 years at a median of 25% (IQR 21%–29%) (Figure 3A). After 10 years, the effectiveness of postexposure vaccines in preventing new infections gradually declined.
In contrast, in terms of reducing the cumulative number of new cases of TB, postexposure vaccines initially had substantially greater effectiveness than preexposure vaccines. After 10 years of vaccination, postexposure vaccines had reduced the cumulative number of TB cases by a median of 34% (IQR 29%–40%) (Figure 3B); effectiveness diminished slightly over the next few decades, despite continuous vaccination of newly infected latent persons (Figure 3B). Preexposure vaccines, despite having reduced the infection rate by 46% (IQR 40%–53%) (Figure 3A), only reduced the cumulative percentage of TB cases by a median of 23% (IQR 21%–25%) after 10 years (Figure 3B). After 20 to 30 years of continuous vaccination, post- and preexposure vaccines had similar effectiveness in terms of the cumulative percent of TB cases prevented (Figure 3B).
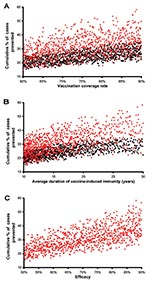
To predict the potential public health impact of pre- and postexposure vaccines, in our uncertainty analysis we varied vaccination coverage rates, duration of vaccine-induced immunity, and vaccine efficacy. We determined the quantitative effect of each of these three variables on the cumulative percentage of TB cases prevented by performing a multivariate sensitivity analysis and calculating partial rank correlation coefficients (PRCCs) (Appendix). The cumulative percentage of TB cases prevented increased substantially (PRCC = 0.93, 0.96) as vaccination coverage rates increased from 60% to 90% (Figure 4A, unadjusted data after 20 years of continuous vaccination); this effect was greater for postexposure vaccines than preexposure vaccines. The cumulative percentage of TB cases prevented also increased substantially (PRCC = 0.95, 0.97) as the average duration of vaccine-induced immunity increased from 10 to 30 years (Figure 4B, unadjusted data after 20 years of continuous vaccination); this effect was greater for postexposure vaccines than preexposure vaccines.
We assessed the effectiveness of 1,000 postexposure vaccines that varied in efficacy from 50% to 90%; vaccine efficacy was defined by the degree of reduction in the disease progression rate of latently infected persons. The cumulative percentage of TB cases prevented increased substantially as the postexposure vaccine efficacy increased from 50% to 90% (Figure 4C, unadjusted data after 20 years of continuous vaccination, PRCC = 0.97). Efficacy of preexposure vaccines is more complex than that of postexposure vaccines; therefore, we modeled the efficacy of preexposure vaccines by three different mechanisms (Figure 2) and evaluated the effect of each of the three mechanisms on the cumulative percentage of TB cases prevented. We assumed that preexposure vaccines could reduce the risk for infection in the uninfected (mechanism 1), allow infection but reduce the probability of rapidly progressing to disease (mechanism 2), and allow infection but reduce the rate of progression of latently infections to disease (mechanism 3). We varied each of these three potential mechanisms independently to vary efficacy levels from 50% to 90%. Preexposure vaccines that operated by using mechanism 3 were not effective (PRCC < 0.5) at preventing a substantial cumulative percentage of TB cases, even if these type of preexposure vaccines had a high efficacy. Preexposure vaccines that operated by either mechanism 1 or 2 were effective in preventing TB cases; with preexposure vaccines that operated by reducing the risk of infection in the uninfected (i.e., mechanism 1) being more effective (PRCC = 0.84) than vaccines that operated by allowing infection but reducing the probability of fast progression to disease (i.e., mechanism 2) (PRCC = 0.66).
We evaluated the potential effectiveness of a variety of pre- and postexposure vaccines in controlling TB epidemics in countries that have both a high incidence of disease and a high prevalence of infection. Under these epidemiologic conditions, we found that preexposure vaccines would be almost twice as effective as postexposure vaccines in reducing the infection rate. In contrast, vaccination campaigns that used postexposure vaccines would initially have a substantially greater effect reducing the number of TB cases than campaigns that used preexposure vaccines. However, our predictions show that (despite continuous vaccination) the effectiveness of campaigns using postexposure vaccines would diminish over time but that the effectiveness of campaigns using preexposure vaccines would increase. Hence, after 20 to 30 years, campaigns using either postexposure or preexposure vaccines would be equally effective (because of the complexity of the vaccine mechanisms that we modeled) in terms of the cumulative number of TB cases prevented.
Since preventing disease is more important than preventing infection and to have an immediate, substantial decrease in TB cases is desirable, our results imply that postexposure vaccines would be more beneficial than preexposure vaccines. Our results show that public health officials should expect campaigns that use postexposure vaccines to first appear highly effective, but that effectiveness will decrease with time. We have also shown that the incidence of disease is likely to remain high even if highly effective vaccines that induce long-term immunity are developed and widely deployed. We found that even widely deployed high-efficacy (50%–90%) pre- or postexposure vaccines are only likely to reduce the number of TB cases by one third. Reductions in the number of TB cases would directly translate into reductions in TB deaths (results not shown). Currently the annual TB death rate is 2 million; hence, our results indicate that the type of vaccines we modeled could save ≈700,000 lives per year. These vaccines could also substantially reduce the emergence of drug-resistant TB (22,24).
To understand why even high-efficacy (50%–90%) vaccines are only capable of reducing the TB death rate by one third, how the natural history of M. tuberculosis infection differs from other, more “simple,” pathogens (e.g., influenza, measles, and smallpox) needs to be examined. For “simple” pathogens, preexposure vaccines can be very effective in reducing epidemic severity because the incidence of disease is a direct function of the incidence of infection. For a “simple” pathogen, if a vaccine reduces infection rates by 80%, then the vaccine will also reduce disease rates by 80%. However, the natural history TB is more complex: the incidence of disease does not directly reflect the incidence of infection with M. tuberculosis. The incidence of disease is driven by two sources: susceptible persons who become infected and quickly progress to disease (source 1) and latently infected persons who slowly progress to disease, often many years after the initial infection (source 2). Both sources make a substantial contribution to the incidence of disease. Preexposure vaccines (given to uninfected persons) will act mainly on reducing the contribution of source 1 to the incidence, but they will have little direct effect on reducing the contribution of source 2. In contrast, postexposure vaccines (given to latently infected persons) will act mainly on reducing the contribution of source 2 to the incidence but will have relatively little effect reducing the contribution of source 1. Therefore, even if highly effective pre- or postexposure vaccines are widely deployed, the incidence of TB in developing countries (as our results show) is likely to remain high.
Also, the increasing HIV epidemic will lead to continuous increases in the incidence of TB in developing countries (26). Currently, what effect co-infection with HIV will have on TB vaccine effectiveness is unclear; possibly, HIV co-infection could reduce vaccine effectiveness. Thus, any new TB vaccine should be evaluated in clinical trials to determine the effect of HIV coinfection on vaccine effectiveness. To reduce the severity of TB epidemics, we recommend that developing and deploying vaccines that act as both pre- and postexposure vaccines are necessary to simultaneously attack both sources that drive the TB rate. Additionally, maintaining high rates of detection and treatment of tuberculosis is necessary, as recommended by the World Health Organization (2); by combining treatment and vaccination strategies, eradicating TB epidemics may be possible, as we have previously shown (16).
Our results have implications for designing both TB vaccines and vaccination campaigns. Highly effective vaccines will be needed to have the public health impact that we have shown (i.e., to reduce the TB death rate by one third). Whether or not the vaccines currently in development will afford this level of efficacy remains to be seen. Moreover, vaccines will need to provide very long-lasting immunity; our current analysis examines the effect of fairly long-lasting vaccines (10–30 years average duration of immunity). Different types of vaccines have different durations of immunity. For example, DNA vaccines should provide lifelong immunity, whereas subunit vaccines will likely require booster vaccinations (28), an approach that would be more logistically difficult and expensive. Also, we have shown that preexposure vaccines are best if they prevent infection (mechanism 1) rather than allow infection but reduce the probability of fast progression to disease (mechanism 2) or reduce the rate of progression of latently infections to disease (mechanism 3). Whether or not new TB vaccines will prevent infection from occurring is not known, but BCG is clearly not able to prevent infection, and vaccines currently in development will likely not be able to do so either (29). As new TB vaccines and other control strategies become available, their potential benefits to TB control efforts can be evaluated by mathematical modeling. Mathematical models can be used as health policy tools to evaluate strategies for controlling TB (30–35); mathematical models also provide insights for predicting the potential public health impact of imperfect HIV vaccines (36–39). Our results show that, because of the complex pathogenic process of TB, high-incidence epidemics are unlikely to be substantially reduced by widely deploying highly effective preexposure or postexposure vaccines. We suggest that to achieve global control of TB, developing a single TB vaccine that functions as both a pre- and a postexposure vaccine is necessary.
Dr. Ziv is an assistant professor of medicine at the University of California, San Francisco. His previous work on tuberculosis includes a model of the population-level efficacy of targeted prevention for early latent infection.
Acknowledgment
S.M.B. gratefully acknowledges financial support of NIH/NIAID (RO1 AI041935).
References
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. DOIGoogle Scholar
- World Health Organization. WHO Report 2002: global tuberculosis control. Surveillance, planning, financing. Geneva: The Organization; 2002.
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. DOIGoogle Scholar
- Behr MA, Wilson ME, Gill WP, Salamon H, Schoolnik GK, Rane S, Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. DOIGoogle Scholar
- Behr MA. BCG—different strains, different vaccines? Lancet Infect Dis. 2002;2:86–92. DOIGoogle Scholar
- McCarthy M. Gates grant boosts tuberculosis vaccine research. US82.0 million dollars grant will double global spending on new tuberculosis vaccines. Lancet. 2004;363:537. DOIGoogle Scholar
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. DOIGoogle Scholar
- Olsen AW, van Pinxteren LAH, Okkels LM, Rasmussen RB, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect Immun. 2001;69:2773–8. DOIGoogle Scholar
- Tascon RE, Colston MJ, Ragno S, Stravropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–92. DOIGoogle Scholar
- Lowrie DB, Tascon RE, Bonato VL, Lima VM, Faccioli LH, Stravropoulos E, Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–71. DOIGoogle Scholar
- Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 20-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97:13853–8. DOIGoogle Scholar
- Ginsberg AM. What’s new in tuberculosis vaccines? Bull World Health Organ. 2002;80:483–8.
- Lietman T, Blower SM. The potential impact of tuberculosis vaccines as epidemic control agents. Clin Infect Dis. 2000;30:S316–22. DOIGoogle Scholar
- Lietman T, Blower SM. Tuberculosis vaccines. Science. 1999;286:1300–1.
- Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev. 1994;2:229–43. DOIGoogle Scholar
- Blower SM, Aschenbach AN, Gershengorn HB, Kahn JO. Predicting the unpredictable: transmission of drug-resistant HIV. Nat Med. 2001;7:1016–20. DOIGoogle Scholar
- Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–4. DOIGoogle Scholar
- Blower SM, Koelle K, Kirschner DE, Mills J. Live attenuated HIV vaccines: predicting the trade-off between efficacy and safety. Proc Natl Acad Sci U S A. 2001;98:3618–23. DOIGoogle Scholar
- Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–21. DOIGoogle Scholar
- Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. DOIGoogle Scholar
- Sanchez MA, Blower SM. Uncertainty and sensitivity analysis of the basic reproductive rate: tuberculosis as an example. Am J Epidemiol. 1997;145:1127–37.
- Porco TC, Blower SM. Quantifying the intrinsic transmission dynamics of tuberculosis. Theor Popul Biol. 1998;54:117–32. DOIGoogle Scholar
- Blower SM, Gerberding JL. Understanding, predicting and controlling the emergence of drug-resistant tuberculosis: a theoretical framework. J Mol Med. 1998;76:624–36. DOIGoogle Scholar
- Porco TC, Small PM, Blower SM. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr. 2001;28:437–44.
- Ziv E, Daley CL, Blower SM. Early therapy for latent tuberculosis infection. Am J Epidemiol. 2001;153:381–5. DOIGoogle Scholar
- Ginsberg AM. A proposed national strategy for tuberculosis vaccine development. Clin Infect Dis. 2000;30(Suppl 3):S233–42. DOIGoogle Scholar
- Letvin NL, Bloom BR, Hoffman SL. Prospects for vaccines to protect against AIDS, tuberculosis, and malaria. JAMA. 2001;285:606–11. DOIGoogle Scholar
- Waaler HT, Piot MA. The use of an epidemiological model for estimating the effectiveness of tuberculosis control measures. Sensitivity of the effectiveness of tuberculosis control measure to the coverage of the population. Bull World Health Organ. 1969;41:75–93.
- Blower SM, Volberding P. What can modeling tell us about the threat of antiviral drug resistance? Curr Opin Infect Dis. 2002;15:609–14.
- Murray CJ, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci U S A. 1998;95:13881–6. DOIGoogle Scholar
- Brewer TF, Heymann SJ, Krumplitsch SM, Wilson ME, Colditz GA, Fineberg HV. Strategies to decrease tuberculosis in US homeless populations: a computer simulation model. JAMA. 2001;286:834–42. DOIGoogle Scholar
- Castillo-Chavez C, Feng Z. Global stability of an age-structure model for TB and its applications to optimal vaccination strategies. Math Biosci. 1998;151:135–54. DOIGoogle Scholar
- Bishai DM, Mercer D. Modeling the economic benefits of better TB vaccines. Int J Tuberc Lung Dis. 2001;5:984–93.
- McLean AR, Blower SM. Imperfect vaccines and herd immunity to HIV. Proc R Soc Lond B Biol Sci. 1993;253:9–13. DOIGoogle Scholar
- Blower SM, McLean AR. Prophylactic vaccines, risk behavior change & the probability of eradicating HIV in San Francisco. Science. 1994;265:1451–4. DOIGoogle Scholar
- Blower SM, Schwartz EJ, Mills J. Forecasting the future of HIV epidemics: the impact of antiretroviral therapies and imperfect vaccines. AIDS Rev. 2003;5:113–25.
- Blower SM, Moss RB, Fernandez-Cruz E. Calculating the potential epidemic-level impact of therapeutic vaccination on the San Francisco HIV epidemic. AIDScience [serial on the Internet]. 2003 Oct;3. Available from: http://www.aidscience.org/Articles/AIDScience040.asp
- Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev. 1994;2:229–43. DOIGoogle Scholar
- Blower SM, Aschenbach AN, Gershengorn HB, Kahn JO. Predicting the unpredictable: transmission of drug-resistant HIV. Nat Med. 2001;7:1016–20. DOIGoogle Scholar
- Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–4. DOIGoogle Scholar
- Blower SM, Koelle K, Kirschner DE, Mills J. Live attenuated HIV vaccines: predicting the trade-off between efficacy and safety. Proc Natl Acad Sci U S A. 2001;98:3618–23. DOIGoogle Scholar
- Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–21. DOIGoogle Scholar
- Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. DOIGoogle Scholar
- Sanchez MA, Blower SM. Uncertainty and sensitivity analysis of the basic reproductive rate: tuberculosis as an example. Am J Epidemiol. 1997;145:1127–37.
- Porco TC, Blower SM. Quantifying the intrinsic transmission dynamics of tuberculosis. Theor Popul Biol. 1998;54:117–32. DOIGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 10, Number 9—September 2004
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sally Blower, Department of Biomathematics, David Geffen School of Medicine at UCLA, 10833 Le Conte Avenue, Los Angeles, CA 90095-1766, USA; fax: 310-206-6116
Top