Volume 11, Number 4—April 2005
Dispatch
Human Spotted Fever Rickettsial Infections
Abstract
Serum specimens from patients at 4 sites in Peru were tested for evidence of spotted fever group rickettsial infection. Results showed that 30 (18%) of 170 patients had spotted fever group rickettsial infections, which likely caused their illnesses. These findings document laboratory-confirmed spotted fever from diverse areas of Peru.
Rickettsial spotted fever was first described in South America in 1931 in Sao Paulo, Brazil (1). The etiologic agent, Rickettsia rickettsii, and the tick vector, Amblyomma cajenennse (the Cayenne tick), were subsequently identified. Serologic evidence of R. rickettsii infections has been documented in several countries in South and central America, including Argentina (2), Brazil and Uruguay (3), Colombia (4), Costa Rica (5), Panama (6), and Mexico (7). A recent study documented for the first time serologic evidence for spotted fever group (SFG) Rickettsia infections in 1 region of northern Peru (8). We describe serologic evidence of SFG rickettsial infections in diverse areas of Peru, including laboratory-confirmed infections among patients with clinical febrile disease.
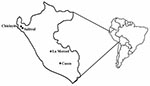
Serum samples were obtained from 4 areas in Peru: Chiclayito and Salitral (Piura Department); La Merced (Junin Department); and Cusco (Cusco Department) (Figure). Chiclayito is a small village (population 6,133) ≈30 m above sea level on the outskirts of the city of Piura in the northern coastal desert. Salitral is a small rural village (population 1,503) ≈162 m above sea level in a more temperate region of the Salitral District (Morropon Province, Piura Department) ≈3 h by car from Chiclayito. La Merced is the capital of the Chanchamayo District (Chanchamayo Province, Junín Department) and located ≈751 m above sea level ≈350 km east of the Peruvian capital city of Lima, on the eastern side of the Andes. The district has a population of 31,000; approximately half live in La Merced. Cusco (population 260,000) is located ≈3,350 m above sea level in the southern Peruvian Andes 1,089 km southeast of Lima.
Sera from patients representing the 4 surveillance sites were tested for antibodies against SFG rickettsiae after written informed consent was provided by each patient (Department of Defense Institutional Review Board No. 31535). Patients enrolled had a fever ≥38ºC and at least 2 other signs or symptoms including headache, myalgia, arthralgia, rash, and bleeding. Patients with a positive blood film for malarial parasites or obvious disease such as diarrhea or upper respiratory illness were excluded.
Paired (acute- and convalescent-phase) patient serum samples were evaluated for immunoglobulin (Ig) G antibodies reactive with R. rickettsii antigen by either an indirect immunofluorescence assay (IFA) or enzyme immunoassay (EIA). Serum specimens were also tested by IFA for typhus group rickettsial antibodies and were uniformly negative. IFA analysis was conducted according to directions provided by the manufacturer (PanBio, INDX, Inc., Baltimore, MD, USA). Endpoint titers were recorded as the reciprocal of the last dilution exhibiting specific fluorescence. Titers ≥1:64 were considered positive. Patients with confirmed spotted fever were those who showed a ≥4-fold increase in R. rickettsii IgG titer from acute phase to convalescent phase of illness.
The EIA was conducted by using a 4-step indirect immunoassay to detect R. rickettsii IgG, as described (8). A positive serum dilution exceeded the mean plus 3 standard deviations between the absorbance of R. rickettsii antigen and the negative control antigen of 5 control serum specimens. Serum samples were titrated to endpoint and the highest dilution found positive was recoded as the R. rickettsii IgG titer. Serum from a serologically confirmed case-patient showed a ≥4-fold increase in antibody titer from the acute to the convalescent phase.
A total of 170 patients, 50 from Chiclayito and the Salitral Health Centers (Piura Department), 67 from Cusco Hospital (Cusco Department), and 53 from La Merced Hospital (Junin Department), were tested for antibodies to SFG rickettsiae. IFA testing was done at the Peruvian National Institute of Health, while EIAs were conducted at Naval Medical Research Center Detachment. Not all patients were tested by both assays (Table 1). Of the 170 patients tested, 30 (18%) yielded results that suggested that SFG rickettsial infections were the most likely cause of their illnesses (Table 1). Patients from all 4 study sites in 3 departments of Peru had evidence of SFG rickettsiae infections as the cause of illness. Frequencies of confirmed patients in the 3 departments did not differ significantly (p > 0.52). Table 2 shows the frequencies of spotted fever by age and sex for the 164 patients for whom data were recorded. Age groups did not differ significantly (p > 0.5). The frequency of spotted fever was 27% in female patients and 10% in male patients (p < 0.005).
The signs and symptoms of patients with confirmed spotted fever who came to the treatment facility included fever and malaise (100%), chills (94%), weakness (94%), shortness of breath (94%), prostration (81%), arthralgia (62%), abdominal pain (62%), cough (56%), nausea (56%), and runny nose (56%). None of the patients died, and most patients had a relatively mild febrile illness. There were no clear clinical differences in patients with confirmed cases of spotted fever compared with febrile patients who did not have spotted fever.
Evidence of SFG rickettsial infection was observed in samples taken from febrile patients in Cusco, Junin, and Piura departments. The etiologic agent or agents responsible for the spotted fever illnesses remain unknown. Appropriate samples from these patients were not available for isolation or molecular identification by a polymerase chain reaction.
Host inflammation may partly contribute to the pathogenic sequelae with intra-endothelial cell infection in more severe SFG infection (9). Patients infected with R. akari typically experience a mild and or asymptomatic disease characterized by low-grade fever, sweats, headache, and a vesicular eruption over the trunk and extremities (10). R. akari is maintained transovarially in the mite vector and transmitted to humans by the house mouse mite (Liponyssoides sanguineus). Infections have generally been reported among higher risk populations such as intravenous drug users (11), or within the densely populated inner city (12). Less is known about the susceptibility of rural agrarian populations. The concentration of humans in close proximity to house mice and their mites are factors that could contribute to an increase in rickettsialpox in the region. Sporadic cases of rickettsialpox may be confused with chickenpox, a common illness associated vesicular rash. However, none of the confirmed SFG rickettsia-infected patients had vesicular rashes typical of rickettsialpox.
Cat flea typhus, caused by R. felis, is a mild disease similar to murine typhus (13). Typical clinical findings include fever, headache, and occasional rash. The clinical manifestations of patients infected with SFG rickettsiae are similar to those described for cat flea typhus. However, recent discoveries of novel rickettsioses caused by distinct SFG rickettsiae in Europe, Africa, Australia, Asia, and North America during the last 25 years (14,15) suggest that the infections reported in this study may be the results of a novel SFG rickettsial agent. Future work is needed to identify the agent involved and to clearly link clinical signs and symptoms with diagnoses.
The higher frequency of cases in women suggests occupational exposure since in these areas of Peru women are generally more involved with domestic activities near the home. Possibilities for increased exposure of women may include more frequent work in the fields, thus exposing them to arthropod vectors; closer contact with domestic animals that may be involved in maintaining the SFG rickettsial agent (although no evidence was collected to support this); or exposure to house mouse mites in the home. Serologic evidence suggests that SFG rickettsiae were responsible for causing febrile illnesses in these 4 study sites of Peru, which demonstrates that SFG rickettsia result in human disease in Peru. Further studies are needed to document the species of SFG rickettsiae and to determine the vectors of these rickettsial infections. In addition, epidemiologic studies are needed to identify the risk factors, document the clinical spectrum, and suggest public health recommendations for prevention.
Dr. Schoeler is a U.S. Navy medical entomologist currently assigned to the Navy Disease Vector Ecology and Control Center in Silverdale, Washington. His research interests include the ecology and control of vectorborne diseases affecting U.S. military forces.
Acknowledgments
We are grateful to Ana-Maria Sanchez for laboratory assistance and Andres G. Lescano for technical assistance.
This investigation was supported by Work Unit Number (WUN) No. 847705 82000 25GB B0016 of the U.S. Navy Global Emerging Infections Surveillance and Response program. The study protocol was approved by the Naval Medical Research Center Institutional Review Board (Protocol No. NMRCD.2000.0006 DoD 31535) in compliance with all Federal regulations governing the protection of human subjects.
References
- Piza J, Salles-Gomes L, Rocha Lima H. Le typhus exanthematique a Sao Paolo. C R Soc Seances Soc Biol Fil. 1931;1106:1020–2.
- Ripoll CM, Remondegui CE, Ordonez G, Arazamendi R, Fusaro H, Hyman MJ, Evidence of rickettsial fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–4.PubMedGoogle Scholar
- Galvao MA, Mafra CL, Moran C, Anaya E, Walker DH. Rickettsiosis of the genus Rickettsia in South America. Ann N Y Acad Sci. 2003;990:57–61. DOIPubMedGoogle Scholar
- Sexton DJ, Muniz M, Corey GR, Breitschwerdt EB, Hegarty BC, Dumler S, Brazilian spotted fever in Esprito Santo, Brazil: description of a focus of infection in a new endemic region. Am J Trop Med Hyg. 1993;49:222–6.PubMedGoogle Scholar
- Fuentes L. Ecological study of Rocky Mountain spotted fever in Costa Rica. Am J Trop Med Hyg. 1986;35:192–6.PubMedGoogle Scholar
- Calero MC, Munez JM, Silva R. Rocky Mountain spotted fever in Panama. Report of three cases. Am J Trop Med Hyg. 1952;1:631–6.PubMedGoogle Scholar
- Bustamante ME, Varela G. Distribucion de las rickettsiasis en Mexico. Rev Inst Salubr Enferm Trop. 1947;8:3–13.
- Blair PJ, Schoeler GB, Moron C, Anaya E, Caceda R, Cespedes M, Evidence of rickettsial and Leptospira infections in Andean northern Peru. Am J Trop Med Hyg. 2004;70:357–63.PubMedGoogle Scholar
- Valbuena G, Feng HM, Walker DH. Mechanisms of immunity against rickettsiae. New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 2002;4:625–33. DOIPubMedGoogle Scholar
- Comer JA, Tzianabos T, Fynn C, Vlahov D, Childs JE. Serologic evidence of rickettsialpox (Rickettsia akari) infection among intravenous drug users in inner-city Baltimore, Maryland. Am J Trop Med Hyg. 1999;60:894–8.PubMedGoogle Scholar
- Paddock CD, Greer PW, Ferebee T, Singleton J Jr, McKechnie DB, Treadwell TA, Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis. 1999;179:1469–76. DOIPubMedGoogle Scholar
- Schriefer ME, Sacci JB Jr, Dumler JS, Bulle MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32:949–54.PubMedGoogle Scholar
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719.PubMedGoogle Scholar
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, Rickettsia parkeri: a newly reported cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–11. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 11, Number 4—April 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
James G. Olson, U.S. Naval Medical Research Center Detachment, Lima, Peru, American Embassy Unit 3800, APO AA 34031-3800; fax: 51-1-561-3042
Top