Volume 13, Number 11—November 2007
Research
Mycobacterium ulcerans in Mosquitoes Captured during Outbreak of Buruli Ulcer, Southeastern Australia
Abstract
Buruli ulcer (BU) occurs in >30 countries. The causative organism, Mycobacterium ulcerans, is acquired from the environment, but the exact mode of transmission is unknown. We investigated an outbreak of BU in a small coastal town in southeastern Australia and screened by PCR mosquitoes caught there. All cases of BU were confirmed by culture or PCR. Mosquitoes were trapped in multiple locations during a 26-month period. BU developed in 48 residents of Point Lonsdale/Queenscliff and 31 visitors from January 2001 through April 2007. We tested 11,504 mosquitoes trapped at Point Lonsdale (predominantly Aedes camptorhynchus). Forty-eight pools (5 species) were positive for insertion sequence IS2404 (maximum likelihood estimate 4.3/1,000), and we confirmed the presence of M. ulcerans in a subset of pools by detection of 3 additional PCR targets.
Buruli ulcer (BU), also known as Bairnsdale ulcer (1), Daintree ulcer (2), and Mossman ulcer in Australia, is an emerging disease of skin and soft tissue with potential to cause scarring and disability (3). It is caused by Mycobacterium ulcerans (4), an environmental pathogen that produces a destructive polyketide toxin, mycolactone (5); the genes for the production of this toxin are encoded on newly described plasmid pMUM001 (6). BU occurs in >30 countries worldwide, but it affects mainly children in sub-Saharan Africa, where it is now more common than tuberculosis and leprosy in some regions (7). This disease occurs in people of all ages and races who live in or visit BU-endemic areas, but the precise mode of transmission remains unknown.
Analysis of the recently sequenced M. ulcerans genome has shown that in addition to pMUM001, there are unusually high copy numbers of 2 independent insertion sequences (IS2404 and IS2606) and a high incidence of pseudogene formation (8). These data suggest that M. ulcerans is unlikely to be free-living in the environment but is instead undergoing adaptation to a specific ecologic niche in which the products of some ancestral genes are no longer essential. One such niche may be in aquatic insects because M. ulcerans has recently been reported to colonize the salivary glands of carnivorous water bugs (Naucoridae) under laboratory conditions (9), and mycolactone production appears to be necessary for this colonization (10). Studies from disease-endemic areas in Africa have reported that farming activities near rivers (11) and swimming in rivers or marshes (12) may be risk factors for BU; bites from contaminated water bugs may transmit the infection.
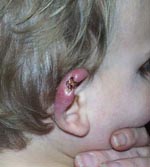
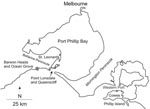
In temperate southeastern Australia, outbreaks of M. ulcerans infection occur in localized areas, but few patients report direct contact with environmental water other than the ocean, which led to the proposal that aerosols from contaminated water may cause human infections (13). However, these low-lying disease-endemic areas also harbor large populations of mosquitoes, and some patients have reported that BU first appeared at the site of what may have been a mosquito bite (Figure 1). These observations, and knowledge from field studies in Africa implicating insects as either a reservoir or mode of transmission, led us to capture and screen mosquitoes during our investigation of a large outbreak of BU in humans in a small coastal town in southeastern Australia (Point Lonsdale), ≈60 km south of Melbourne (Figure 2).
Outbreak Investigation
M. ulcerans infection has become increasingly common in the southern Australian state of Victoria since the early 1990s (14,15) and characteristically causes localized outbreaks (16). In 1995, a research group at the Royal Children’s Hospital in Melbourne developed an IS2404 PCR to improve speed and accuracy of diagnosis of BU (17). This method has now become the initial diagnostic method of choice in Australia and elsewhere (18). All PCR- and culture-positive cases of M. ulcerans infection in Victoria have been unofficially reported to the Victorian Department of Human Services (DHS) since the 1990s, and investigators from DHS began to routinely contact and interview all new reported case-patients in 2000. All new cases of M. ulcerans infection were made legally reportable in Victoria in January 2004 (19).
Case Definition
For this study, a case of BU was defined as a patient with a suggestive clinical lesion from which M. ulcerans was identified by PCR or culture from a swab or tissue biopsy specimen from January 2002 through April 2007; the patient must have been either a resident of, or a visitor to, Point Lonsdale or Queenscliff (adjacent coastal towns on the Bellarine Peninsula) who did not report a recent history of contact with another known BU-endemic area. Australian Bureau of Statistics data derived from the 2001 Australian Census for Point Lonsdale/Queenscliff (postcode 3225) were used to obtain the resident population numbers and age distribution in the outbreak area (20).
Mosquito Trapping
A total of 8–13 overnight mosquito traps were placed at Point Lonsdale on 22 occasions from December 2004 through January 2007. Adult mosquito sampling was conducted with CO2-baited miniature light traps (21). Traps were 2-L, cylindrical, insulated containers designed to hold CO2 pellets that continuously produce CO2, which then diffuses through holes in the bottom of the container. A small electric light and fan at the base of the container deflected attracted mosquitoes into a holding container. The traps were set before dusk and then retrieved several hours after dawn the next morning. The catches were transported to Primary Industries Research in Attwood, Victoria, where they were counted, sorted, and pooled by sex and species. Mosquito species were identified by using the key of Russell (22). All captured mosquitoes were tested except in February–March 2005 and again in October 2005 when recent rains led to large spikes in mosquito numbers.
Screening of Mosquitoes by PCR
DNA was extracted from pools of <15 individual mosquitoes (occasional pools had <23 mosquitoes) of the same sex and species by using the FastDNA Kit (revision no. 6540-999-1D04) and the FastPrep Instrument (Qbiogene Inc., Irvine, CA, USA) according to the manufacturer’s instructions. We adapted fluorescence-based real-time PCR technology to screen mosquitoes for 3 M. ulcerans–specific DNA sequences as described (23). Briefly, oligonucleotide primers and TaqMan MGB probes (Applied Biosystems, Foster City, CA, USA) labeled with fluorescent dyes 6FAM or VIC were designed that targeted 3 independent high-copy number repetitive sequences (IS2404 and IS2606 [24] and the ketoreductase B domain [KR] from pMUM001 [6]). The copy number of these targets per bacterial cell in the published sequenced of M. ulcerans, to which the outbreak strain is phylogenetically closely related, is 213 for IS2404, 91 for IS2606, and 30 for KR (8). Assays were conducted with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems).
Each pool was first tested for IS2404 with an internal positive control to test for PCR inhibition and separate negative and positive controls. Samples were considered positive for a given target when they had a result above a previously determined critical threshold (23). Pools that were positive for IS2404 were then screened in duplicate with confirmatory assays to detect IS2606 and KR. For pools with sufficiently high signal strength, amplification and sequencing of variable number tandem repeat (VNTR) locus 9 were conducted by using a nested PCR. The first round PCR used 2 primers, MUVNTR9NF (5′-ACTGCCCAGACATGGCGA-3′) and MUVNTR9NR (5′-ACGCGAGGTGGAACAAAGC-3′), designed to flank the published VNTR locus 9 primer. First-round PCR products were used as template for a second-round PCR performed as described by Ablordey et al. (25). PCR products of the expected size were sequenced by using the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer’s instructions. Precipitated reaction products were tested in a 3730S Genetic Analyzer (Applied Biosystems) (23). The maximum likelihood estimate (MLE) per 1,000 mosquitoes tested (bias corrected MLE) was calculated by using software recommended for this purpose by the Centers for Disease Control and Prevention (Atlanta, GA, USA) (26).
Description of Outbreak
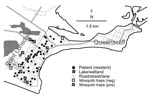
The climate in Point Lonsdale is temperate with a mean daily maximum temperature of 12.8°C in July (winter) and 22.4°C in January (summer). Average annual rainfall is 660 mm and is spread throughout the year (e.g., average 41.3 mm in January and 59.1 mm in July) (27). Most of the town is low-lying and close to sea level, and there are several natural and human-made swamps and water features in the vicinity (Figure 3). Natural vegetation includes dense clumps of coastal tea trees (Leptospermum laevigatum).
Point Lonsdale shares a beach with Queenscliff (Figure 3), a neighboring town 4 km to the east. Point Lonsdale and Queenscliff (postcode 3225) were included in the 2001 Australian Bureau of Statistics Census and had a resident population of 3,851 at that time, but there are also large numbers of visitors to this scenic area during summer holiday periods (e.g., 54,000 people visited the Queenscliff Visitor Information Centre in 2005; pers. comm.).
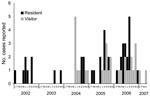
M. ulcerans infection was not found in the area before 2002. From January 2002 through April 2007, BU developed in 79 persons (48 residents and 31 visitors). Initially, all patients were local residents, but in 2004 the outbreak increased in intensity and began to include visitors as well as residents (Figure 4). All case-patients who could be accurately located either lived in or visited Point Lonsdale and the western edge of Queenscliff, and none were linked solely to the main township of Queenscliff.
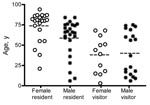
Most case-patients were adults and many were elderly (Figure 5), although 14 of the 79 were children <18 years of age. Among visitors, there was a bimodal age distribution, with relatively low numbers of adults 20–50 years of age. An estimate of the age-specific attack rate for residents of Point Lonsdale/Queenscliff was obtained with reference to the 2001 Australian census. Because census data were not available for the 2 towns separately, the calculation assumes that the age distribution of Point Lonsdale and Queenscliff is similar. A similar analysis for visitors was not performed because appropriate denominators could not be determined. The risk appeared to increase strongly with age and was ≈7× higher for those >55 years of age than in those <55 years of age (p<0.001) (Figure 6).
The incubation period for residents and for most visitors could not be assessed because exposure to the BU-endemic area occurred repeatedly over a prolonged period. However, in 2006, one patient reported a 1-week contact history with Point Lonsdale (P.D.R. Johnson, unpub. data). Her case was diagnosed and reported 7 months after this exposure.
Mosquito Testing
A total of 23,692 mosquitoes were captured in Point Lonsdale during a 25-month period; 96% were Aedes camptorhynchus (Thomson). Twelve other species comprised the remaining 4% (Table 1). Of 11,504 mosquitoes tested, 48 pools were positive for IS2404; of these, 13 pools were also positive for KR and IS2606. Forty-one of 48 pools were female Ae. camptorhynchus, 4 were positive pools of Coquillettidia linealis (Skuse), and 1 each were Anopheles annulipes (Walker s.l.), Culex australicus (Dobrotworsky and Drummond), and Ae. notoscriptus (Skuse). For 2 positive pools with particularly high M. ulcerans DNA concentrations, VNTR locus 9 was amplified and the sequence was identical to that of the local outbreak strain cultured from case-patients (23).
Thirty-five IS2404-positive pools did not contain IS2606 and KR. However, the cycle threshold (Ct) values for IS2404 were lower for those pools that did have IS2606 and KR, which suggested that failure to detect KR and IS2606 in some pools was caused by low DNA concentration, rather than lack of specificity for M. ulcerans. This finding is consistent with known differences in copy number per cell of targets used for PCR screening and confirmation (23). A total of 124 pools of mosquitoes that were negative for IS2404 by PCR were screened with probes for KR and IS2606. None were positive, which indicated that these 2 loci are consistently linked to IS2404 and do not occur independently.
The MLE (bias corrected) for all mosquitoes over the entire testing period at Point Lonsdale was 4.3 M. ulcerans PCR-positive mosquitoes/1,000 tested (95% confidence interval [CI] 3.2–5.6). However, mosquito numbers varied widely between trappings, as did proportions of positive pools. On 1 occasion, only 269 mosquitoes were trapped, but 6 of the pools were positive (December 2005; MLE 22.4, 95% CI 10.3–50.3). Most PCR-positive pools had relatively high Ct values for IS2404 PCR, which indicated low numbers of contaminating M. ulcerans cells. With reference to spiking experiments under laboratory conditions, ≈10–100 M. ulcerans were likely to have been present per contaminated mosquito (23).
Mosquito Numbers, Proportion PCR Positive, and Reporting of BU
Trapping was conducted at Point Lonsdale between December 2004 and January 2007. Mosquito numbers varied during the period, and traps were not set when local reports suggested low mosquito numbers (Appendix Figure). There appeared to be a qualitative relationship between PCR-positive mosquitoes in spring and summer (September–February) and reporting of new cases of human disease in autumn and winter (March–August). The exposure-to-reporting interval is typically longer than the actual incubation period because patients do not always seek medical assistance immediately and doctors do not always diagnose BU when a patient is first seen (28).
Mosquitoes Caught at Other Locations in Victoria
To test that the observed association between M. ulcerans and mosquitoes only occurs in outbreak areas, we tested 3,385 mosquitoes from several inhabited areas with lower BU endemicity than Point Lonsdale. From October 2005 through January 2007, a total of 2,119 mosquitoes (89% Ae. camptorhynchus) were trapped in townships on the Bellarine Peninsula where 30 cases of BU have been reported in the past 5 years; 3 pools of Ae. camptorhynchus were positive by IS2404 PCR. In January and June 2006, a total of 795 mosquitoes (82% Ae. camptorhynchus) were trapped in the Bass Coast Shire, which includes Phillip Island, a region that has previously been endemic for M. ulcerans (14) but has only reported 2 cases in the past 5 years. One pool of Ae. notoscriptus was positive for IS2404. From February through April 2006, 471 mosquitoes were captured from inhabited areas in northern and central Victoria where no human cases of M. ulcerans have been reported. Ten different species were trapped, including 226 Ae. camptorhynchus (48%), but all pools were negative for IS2404. When analyzed together, an association was observed between degree of endemicity and probability of trapping mosquitoes that are positive by PCR for M. ulcerans (Table 2), but this association did not show statistical significance (p = 0.07).
To our knowledge, the outbreak of BU in Point Lonsdale is the largest ever recorded in Australia and has now caused more than twice as many cases as the well-described outbreak at Phillip Island a decade earlier (16,29). A striking feature of both outbreaks is their intensely localized nature. We identified 79 cases that were epidemiologically linked to Point Lonsdale and the western edges of Queenscliff, but the town of Queenscliff, only 4 km to the east along the same beach, has so far remained disease free. The cumulative attack rate for both towns is estimated to be 1.2% of the resident population, but it could be up to twice as high if only the population of Point Lonsdale, where all transmission appears to have occurred, were considered. Although Queenscliff remains unaffected, the nearby towns of Barwon Heads and Ocean Grove, ≈12 km west of Point Lonsdale, began reporting their first cases in 2005.
The first case at Point Lonsdale was reported in January 2002. In 2004, the outbreak increased in intensity and began to involve visitors as well as residents, which suggested that environmental contamination with M. ulcerans has steadily increased over 5 years. Among local residents, we found a higher attack rate in the elderly, with 3.7% of residents of Point Lonsdale/Queenscliff >75 years of age with BU. The reasons for this age distribution are not known, but increasing risk with age could be caused by an age-related immune defect or an unrecognized behavioral factor. Among visitors, there was a pronounced bimodal age distribution, which probably represents a skewing of the exposed population (e.g., young children going to stay with their retired grandparents over the summer while their parents stayed at work) but may also reflect increased susceptibility in young persons. This bimodal pattern, which included increased incidence in young persons and the elderly, has also been reported in Africa (30).
During our investigations at Point Lonsdale, we focused initially on several marshy areas and obtained positive PCR results for plant material from 2 small ornamental lakes and soil from storm water drains (23). However, case-patients did not report direct contact with these lakes or drains (these sources of water are not used for swimming or wading). Thus, how people were exposed is not clear. In an outbreak in Phillip Island, many cases were clustered around a newly formed wetland and a golf course irrigation system, and we proposed transmission from these sites by aerosol (16,29). However, this hypothesis may not be supported by our new evidence, which suggests that M. ulcerans may not be free-living in the environment but may have adapted to specific niches within aquatic environments, including salivary glands of some insects. Thus, we investigated whether M. ulcerans could be detected in mosquitoes, which had been reported in higher than usual numbers at Point Lonsdale. We also investigated behavior in a case-control study (the subject of a separate report), which found that being bitten by mosquitoes increased the odds of having BU (31).
A total of 14,889 mosquitoes obtained over a 25-month period (11,504 from Point Lonsdale) were tested for M. ulcerans by using a highly sensitive and specific real-time PCR (23). We used PCR because direct culture of M. ulcerans from the environment is extremely difficult and was only achieved when IS2404 PCR screening of environmental samples accurately directed researchers to specific microenvironments that include water insects and aquatic plants (32). Although IS2404, IS2606, and the mycolactone-producing virulence plasmid have been detected in mycobacteria other than M. ulcerans (33–35), identification of these targets in expected relative proportions and the VNTR locus 9 sequence identical to that of the outbreak strain in a subset of mosquito pools with sufficiently high DNA concentrations confirms that we identified the outbreak strain (23).
We also demonstrated that over a 2-year cycle at Point Lonsdale absolute numbers of mosquitoes and PCR-positive mosquitoes increased in spring and summer followed by a cluster of new human cases in autumn and winter. This pattern is consistent with recent point estimates that suggest the incubation period for BU in Australia is 3–7 months (2 cases) (36) and 1–4 months (3 cases) (28), and that an additional 1–6 weeks may elapse before cases are diagnosed and reported (28).
The predominant species trapped was Ae. camptorhynchus; however, identification of M. ulcerans in 4 other species suggests that M. ulcerans contamination of mosquitoes is not species specific. Ae. camptorhynchus is a salt marsh species, an aggressive biter, and a major pest in coastal areas of southeastern Australia that has been linked to transmission of Ross River virus. The mosquito appears in large numbers after rain as minimum temperatures begin to increase, with a lag time of ≈1 month (37). Of the other species from which at least 1 PCR-positive pool was identified, An. annulipes and Cq. linealis are fresh water species (38). Ae. notoscriptus is a peridomestic species that breeds in containers (e.g., in roof gutters) (39), can transmit dog hookworm, and has a limited flight range (e.g., <200 m) (40). In contrast, Cx. australicus may have a flight range of many kilometers (41). A limited number of other biting or aquatic insects were also tested and none were positive for M. ulcerans. However, larger numbers must be screened before it can be concluded that they do not transmit M. ulcerans.
Our results do not demonstrate viability or transmissibility of M. ulcerans at the time mosquitoes were captured, and the method we used does not answer questions about location of M. ulcerans within the insect. Because M. ulcerans is an environmental pathogen, PCR-positive mosquitoes may only be indicators of its presence in the environment and not linked to transmission. The Ct values obtained for mosquito pools suggest that only 10–100 organisms were present per positive pool, which is more consistent with organisms being acquired on outer surfaces of mosquitoes when resting or feeding in storm water drains (23), rather than mosquitoes being a true productive reservoir and vector. However, if some bacterial cells were present on the proboscis, they could have been injected beneath the keratin layer during feeding. Although the inoculum size required to cause a human infection is unknown, the long incubation period suggests a low initial inoculum. Our findings do not demonstrate that mosquitoes are responsible for transmission, but this possibility should be investigated. Studies are underway to artificially infect mosquito larvae with M. ulcerans and initiate infection in a mouse model, as has been conducted with naucorids (9).
Although our findings may not apply to the situation in Africa, the close genetic relationship of Australian isolates of M. ulcerans with strains from humans with BU in Africa (35) should encourage similar search on M. ulcerans in mosquitoes from the primary BU-endemic regions of West Africa. We have shown that a small proportion of mosquitoes of 5 species captured in a BU-endemic area during an intense human outbreak of BU can carry M. ulcerans; PCR-positive mosquitoes are likely present at times of peak transmission and mosquitoes captured in areas with few human cases appear less likely to be positive for M. ulcerans. We hypothesize that transmission by mosquitoes offers a partial explanation for the outbreak at Point Lonsdale and possibly at other sites in southeastern Australia.
Dr Johnson is deputy director of the Infectious Diseases Department at Austin Health, a University of Melbourne teaching hospital. His research interests include epidemiology, transmission, and treatment of M. ulcerans infections, drug resistance in Staphylococcus aureus, and hospital infection control.
Acknowledgment
This study was supported by the Victorian State Government Department of Human Services Public Health research grant (2004-7).
References
- Jenkin GA, Smith M, Fairley M, Johnson PD. Acute, oedematous Mycobacterium ulcerans infection in a farmer from far north Queensland. Med J Aust. 2002;176:180–1.PubMedGoogle Scholar
- Johnson PD, Stinear TP, Small PL, Pluschke G, Merritt RW, Portaels F, Buruli ulcer (Mycobacterium ulcerans): new insights, new hope for disease control. PLoS Med. 2005;2:e108. DOIPubMedGoogle Scholar
- MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93–122. DOIPubMedGoogle Scholar
- George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–7. DOIPubMedGoogle Scholar
- Stinear TP, Mve-Obiang A, Small PL, Frigui W, Pryor MJ, Brosch R, Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc Natl Acad Sci U S A. 2004;101:1345–9. DOIPubMedGoogle Scholar
- Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Guedenon A, Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg Infect Dis. 2004;10:1391–8.PubMedGoogle Scholar
- Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17:192–200. DOIPubMedGoogle Scholar
- Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, Legras P, Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol. 2002;68:4623–8. DOIPubMedGoogle Scholar
- Marsollier L, Aubry J, Coutanceau E, Andre JP, Small PL, Milon G, Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol. 2005;7:935–43. DOIPubMedGoogle Scholar
- Marston BJ, Diallo MO, Horsburgh CR Jr, Diomande I, Saki MZ, Kanga JM, Emergence of Buruli ulcer disease in the Daloa region of Côte d’Ivoire. Am J Trop Med Hyg. 1995;52:219–24.PubMedGoogle Scholar
- Aiga H, Amano T, Cairncross S, Adomako J, Nanas OK, Coleman S. Assessing water-related risk factors for Buruli ulcer: a case-control study in Ghana. Am J Trop Med Hyg. 2004;71:387–92.PubMedGoogle Scholar
- Hayman J. Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol. 1991;20:1093–8. DOIPubMedGoogle Scholar
- Johnson PD, Veitch MG, Leslie DE, Flood PE, Hayman JA. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust. 1996;164:76–8.PubMedGoogle Scholar
- Johnson PD, Hayman JA, Quek TY. Fyfe JA, Jenkin GA, Buntine JA, et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust. 2007;186:64–8.PubMedGoogle Scholar
- Veitch MGK, Johnson PDR, Flood PE, Leslie DE, Street AC, Hayman JA. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol Infect. 1997;119:313–8. DOIPubMedGoogle Scholar
- Ross BC, Marino L, Oppedisano F, Edwards R, Robins-Browne RM, Johnson PD. Development of a PCR assay for the rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol. 1997;35:1696–700.PubMedGoogle Scholar
- Phillips R, Horsfield C, Kuijper S, Lartey A, Tetteh I, Etuaful S, Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of Buruli ulcer. J Clin Microbiol. 2005;43:3650–6. DOIPubMedGoogle Scholar
- Victorian Department of Human Services. Notifications of infectious diseases: Summary reports [cited 2007 Aug 18]. Available from http://www.health.vic.gov.au/ideas/downloads/daily_reports/rptvictoriansummary.pdf
- Australian Bureau of Statistics 2001 census [cited 2007 Aug 28]. Available from http://www.abs.gov.au
- Rohe DL, Fall RP. A miniature battery powered CO2 baited light trap for mosquito-borne encephalitis surveillance. Bulletin of the Society of Vector Ecology. 1979;4:24–7.
- Russell RC. A colour photo atlas of mosquitoes of southeastern Australia. 1996 [cited 2007 Aug 17]. Available from http://medent.usyd.edu.au/mosqkey/mosquito_key.htm#8a
- Fyfe JA, Lavender CJ, Johnson PD, Globan M, Sievers A, Azuolas J, Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol. 2007;73:4733–40. DOIPubMedGoogle Scholar
- Stinear T, Ross BC, Davies JK, Marino L, Robins-Browne RM, Oppedisano F, Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37:1018–23.PubMedGoogle Scholar
- Ablordey A, Swings J, Hubans C, Chemlal K, Locht C, Portaels F, Multilocus variable-number tandem repeat typing of Mycobacterium ulcerans. J Clin Microbiol. 2005;43:1546–51. DOIPubMedGoogle Scholar
- Biggerstaff B. Software for mosquito surveillance. Atlanta: Centers for Disease Control and Prevention [cited 2007 Aug 17]. Available from http://www.cdc.gov/ncidod/dvbid/westnile/software.htm
- Australian Bureau of Meteorology [cited 2007 Aug 17]. Available from http://www.bom.gov.au/climate/averages/tables/cw_087100.shtml
- Quek TY, Henry MJ, Pasco JA, O’Brien DP, Johnson PD, Hughes A, Mycobacterium ulcerans infection: factors influencing diagnostic delay. Med J Aust. In press.
- Ross BC, Johnson PDR, Oppedisano F, Marino L, Sievers A, Stinear T, Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997;63:4135–8.PubMedGoogle Scholar
- Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Scott JT, Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop Med Int Health. 2004;9:1297–304. DOIPubMedGoogle Scholar
- Quek TYJ, Athan E, Henry MJ, Pasco JA, Redden-Hoare J, Hughes A, Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis. 2007;13:1661–6.PubMedGoogle Scholar
- Marsollier L, Stinear T, Aubry J, Saint Andre JP, Robert R, Legras P, Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol. 2004;70:1097–103. DOIPubMedGoogle Scholar
- Rhodes MW, Kator H, McNabb A, Deshayes C, Reyrat JM, Brown-Elliott BA, Mycobacterium pseudoshottsii sp. nov., a slowly growing chromogenic species isolated from Chesapeake Bay striped bass (Morone saxatilis). Int J Syst Evol Microbiol. 2005;55:1139–47. DOIPubMedGoogle Scholar
- Ranger BS, Mahrous EA, Mosi L, Adusumilli S, Lee RE, Colorni A, Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect Immun. 2006;74:6037–45. DOIPubMedGoogle Scholar
- Yip MJ, Porter JL, Fyfe JA, Lavender CJ, Portaels F, Rhodes M, Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J Bacteriol. 2007;189:2021–9. DOIPubMedGoogle Scholar
- Lavender CJ, Senanayake SN, Fyfe JA, Buntine JA, Globan M, Stinear TP, First case of Mycobacterium ulcerans disease (Bairnsdale or Buruli ulcer) acquired in New South Wales. Med J Aust. 2007;186:62–3.PubMedGoogle Scholar
- Barton PS, Aberton JG, Kay BH. Spatial and temporal definition of Ochlerotatus (Aedes) camptorhynchus (Thomson) (Diptera: Culicidae) in the Gippsland Lakes system of eastern Victoria. Aust J Entomol. 2004;43:16–22. DOIGoogle Scholar
- Russell RC, Cloonan MJ, Wells PJ, Vale TG. Mosquito (Diptera: Culicidae) and arbovirus activity on the south coast of New South Wales, Australia, in 1985–1988. J Med Entomol. 1991;28:796–804.PubMedGoogle Scholar
- Montgomery BL, Ritchie SA. Roof gutters: a key container for Aedes aegypti and Ochlerotatus (Aedes) notoscriptus (Diptera: Culicidae) in Australia. Am J Trop Med Hyg. 2002;67:244–6.PubMedGoogle Scholar
- Watson TM, Saul A, Kay BH. Aedes notoscriptus (Diptera: Culicidae) survival and dispersal estimated by mark-release-recapture in Brisbane, Queensland, Australia. J Med Entomol. 2000;37:380–4. DOIPubMedGoogle Scholar
- Kay BH, Farrow RA. Mosquito (Diptera: Culicidae) dispersal: implications for the epidemiology of Japanese and Murray Valley encephalitis viruses in Australia. J Med Entomol. 2000;37:797–801. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 11—November 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Paul D.R. Johnson, Infectious Diseases Department, Austin Health, PO Box 5555, Heidelberg 3084, Melbourne, Victoria, Australia;
Top