Volume 15, Number 10—October 2009
Dispatch
West Nile Virus Infection in Plasma of Blood and Plasma Donors, United States
Figure 2
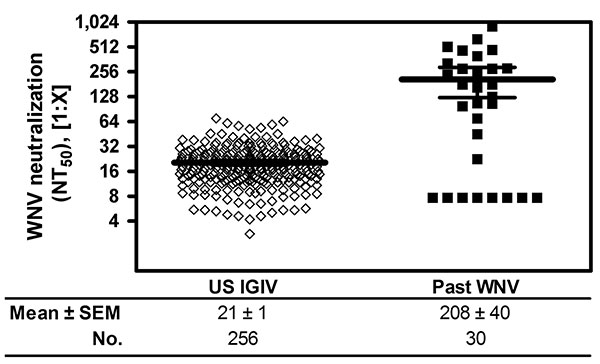
Figure 2. West Nile virus (WNV) neutralization by US plasma-derived immune globulin intravenous (human) (IGIV) released in 2008 and plasma from donors with past WNV infection (past WNV), confirmed by nucleic acid testing. WNV neutralization titers are shown as the mean ± SEM (limit of detection <0.8 for undiluted IGIVs and <7.7 for prediluted sera). NT50, 50% neutralization titer.
Page created: December 07, 2010
Page updated: December 07, 2010
Page reviewed: December 07, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.