Volume 15, Number 7—July 2009
Dispatch
Methicillin-Resistant Staphylococcus aureus ST398 in Swine Farm Personnel, Belgium
Abstract
We assessed methicillin-resistant Staphylococcus aureus (MRSA) in persons on 49 swine farms in Belgium. Surveys showed that 48 (37.8%) persons carried MRSA ST398 and 1 (0.8%) had concurrent skin infection. Risk factors for carriage were MRSA carriage by pigs, regular contact with pigs and companion animals, and use of protective clothing.
Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) carriage has been high (>10%) among swine and exposed farmers and veterinarians (1,2). These MRSA strains are genetically unrelated to hospital- or community-acquired clones. They are resistant to digestion with SmaI and belong to ST398 (1). We assessed prevalence and characteristics of MRSA carriage and infection and associated risk factors for swine farm residents and workers in Belgium during 2007.
We randomly selected 50 farms from the 7,500 farms in the pig farm national database of the Belgian Federal Agency for Food Safety. Sample size was based on an estimated 20% prevalence of MRSA colonization among farm workers and residents (1). The sample size was calculated to test the null hypothesis of prevalence <10% with a power of 80%. Participation was offered to all persons on the farm at the time of the visit, including farmers, co-workers, and household members. After giving written consent, participants were screened for MRSA carriage and interviewed. Simultaneously, on the same farms, a study of MRSA carriage in 30 randomly selected pigs per farm was conducted by veterinarians (3).
Samples from anterior nares and skin lesions on hands or face of human participants were placed into Stuart transport medium (Copan, Italy), inoculated within 24 h into 7.5% NaCl brain–heart infusion enrichment broth, and subcultured after 24 h onto Chromagar MRSA (bioMérieux, Marcy l’Etoile, France) and mannitol salt agar (Becton Dickinson, Heidelberg, Germany). S. aureus isolates were identified by coagulase test and PCR for 16S rRNA, mecA, and nuc genes (4).
Isolates were genotyped by pulsed-field gel electrophoresis after SmaI macrorestriction, spa sequence typing (http://spaserver.ridom.de), and determination of staphylococcal cassette chromosome mec (SCCmec) type and accessory gene regulator (agr) polymorphism (4,5). Four MRSA isolates were further analyzed by multilocus sequence typing (www.mlst.net). Multiplex PCR was used to test for Panton-Valentine leukocidin, toxic shock syndrome toxin 1, and exfoliatin A and B genes (6).
Antimicrobial drug susceptibility was tested by the Vitek2 system (bioMérieux). Multiplex PCR was used to test for resistance genes tetK, tetM, aac(6′)-Ie + aph(2′′), ant(4′)-Ia, aph(3′)-IIIa, ermA, and ermC (7–9).
Data were analyzed by using Stata 9.2 (Statacorp, College Station, TX, USA). We calculated prevalence of MRSA carriage in humans and 95% confidence intervals (CIs) by using cluster survey analysis. We performed risk factor analysis by using multiple logistic regression for cluster surveys, adjusting for clustering within farms. p values <0.05 were considered significant.
From April through July 2007, veterinarians investigated 50 swine farms. Of 1,500 pigs from 34 farms, 663 (44.2%) carried MRSA (3). On 49 of these 50 farms, 127 persons agreed to participate. Nasal (127) and wound (5) swabs showed that 48 (37.8%, 95% CI 25.6%–50.0%) participants carried MRSA and 22 (17%, 95% CI 10.7%–23.9%) carried methicillin-susceptible S. aureus (MSSA). One (2%) of the nasal MRSA carriers had a hand lesion infected with MRSA ST398 and treated it with topical antiseptic. Cultures from wounds on 4 other participants were negative for MRSA. Carriers of MRSA and MSSA were found on 25 (51.0%) and 15 (30.6%) farms, respectively. Prevalence of MRSA carriage was 50% for participants on farms with MRSA-colonized pigs versus 3% on farms without colonized pigs (relative risk 16.5, 95% CI 2.4–114.9, p <0.001).
Univariate analysis showed MRSA carriage to be associated with being a farmer or farm co-worker, being male, having regular contact with animals (including goats, sheep, dogs, or cats) and, paradoxically, wearing gloves and apron and reporting occasional or regular hand disinfection with an antimicrobial product. Multivariate analysis showed MRSA carriage to be independently associated with MRSA prevalence among pigs at the farm, being a farmer with regular pig contact, reporting regular contact with dogs and horses, and reporting use of protective clothing (apron, gloves, or mask) (Table 1).
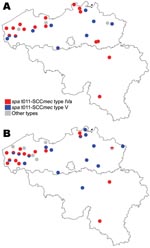
The 48 MRSA isolates were nontypeable by SmaI; some harbored SCCmec type IVa (n = 26), type V (n = 20), or were nontypeable (n = 2), and some exhibited 3 related spa types, t011 (n = 45), t034 (n = 2), and t567 (n = 1) (Table 2). Of the spa types, 4 representative strains belonged to ST398. Of the strains, 94% were classified into 2 genotypes, t011-SCCmec type IVa and t011-SCCmec type V, each found on 14 and 10 farms, respectively. In 8 of 11 farms with >2 MRSA carriers, all carriers harbored the same spa-SCCmec genotype. On 17 of 24 farms with MRSA colonization of humans and pigs, both groups carried the same genotype, suggesting animal-to-human transmission (Figure 1). Of MRSA isolates, 40 (83%) were resistant to tetracycline, cotrimoxazole, macrolides-lincosamides, aminoglycosides, and ciprofloxacin (Figure 2). Nearly all strains were susceptible to fusidic acid and mupirocin; all were susceptible to linezolid, rifampin, and glycopeptides. Resistance to aminoglycosides was conferred by the aac(6′)–aph(2′′) gene (n = 23) and the ant(4′) gene (n = 9). Resistance to macrolides-lincosamides was mainly mediated by ermC gene (n = 24). Tetracycline resistance was encoded by tetM and tetK genes in 48 (100%) and 23 (50%) isolates, respectively. Resistance profiles were related to clonal types (Table 2).
MSSA isolates belonged to spa type t011 or t034 corresponding to ST398 (n = 3) and to 7 PGFE types and 12 spa types (n = 19). Genes encoding toxic shock syndrome toxin 1 (n = 4) and exfoliatin A (n = 3) were detected in 7 MSSA isolates. MSSA isolates were susceptible to all antimicrobial drugs except tetracycline (Figure 2).
Human carriage of MRSA was associated with swine colonization with MRSA. Prevalence rate (38%) was higher than that for hospitalized patients or nursing home residents in Belgium (www.nsih.be/surv_mrsa/download_fr.asp). MRSA isolates from farmers belonged to closely related spa types corresponding to ST398, which are unrelated to hospital- and community-acquired strains but identical to strains from humans in contact with pigs in other European countries (1,2,10).
Despite the high prevalence of nasal MRSA, active MRSA skin infection was detected infrequently (<1%), within the range described in recent US-based studies (11). In a hospital in the Netherlands, a lower attack rate was found for MRSA ST398 than for other MRSA strains (12). However, invasive infections caused by MRSA ST398 have been reported, suggesting that this genotype is pathogenic for humans (2). In our study, MRSA strains did not harbor exotoxin.
Two MRSA genotypes were predominant. For 70% of farms with multiple MRSA carriers, all strains belonged to the same genotype, suggesting transmission within the farm. Although these strains have been shown to not spread easily in hospitals (12), outbreaks of MRSA ST398 in a residential care facility and a hospital probably originated from healthcare workers living on pig farms (13,14). In contrast with MRSA strains, MSSA isolates in our study showed diverse genotypes that frequently colonize human populations (4). MSSA isolates from 3 farmers belonged to the ST398 genotype, which is infrequently reported in humans except in pig farmers with contact with pigs (4).
Risk factors for MRSA ST398 carriage included regular contact with pigs but also with horses and dogs (10), suggesting that different animals could be MRSA ST398 reservoirs or vectors, at least on pig farms. Protective measures did not seem to reduce the risk of becoming colonized with MRSA; this lack of effectiveness has previously been observed for veterinarians (15). This apparent lack of protection should be further investigated to determine routes of transmission other than direct contact with pigs, including airborne transmission and contact with contaminated surfaces and companion animals.
Dr Denis is a clinical microbiologist in the Belgian National Reference Laboratory for MRSA, Université Libre de Bruxelles Hôpital Erasme. His primary research interest is the epidemiology of MRSA.
Acknowledgments
We thank Dimitri Arijs, Frans Castryck, and Paul Heylen (deceased) for collecting animal samples; Eva Leens and Sophie Quoilin for field assistance with the farmer survey; Ariane Deplano, Raf De Ryck, Claire Nonhoff, Sébastien Crevecoeur, and Sylvianne Rottiers for laboratory assistance; Michiel Costers and Herman Claeys for logistic support; and Lucie Seyler for reviewing the manuscript.
This study was supported by the Belgian Antibiotic Policy Coordination Committee, the Federal Public Health Service, and the Sanitary Fund against Porcine Diseases. The study protocol was based on the protocol of a similar survey by the Dutch Working Party on Surveillance and Research of MRSA, kindly provided by Edine Tiemersma and Jan Kluytmans.
References
- Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11:1965–6.PubMedGoogle Scholar
- Witte W, Strommenger B, Stanek C, Cuny C. Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg Infect Dis. 2007;13:255–8.PubMedGoogle Scholar
- Willems G, Dispas M, Denis O, Hallin M, Suetens C, Gordts B, Characterisation of MRSA from pigs in Belgium. Abstract of 2nd Symposium on Antimicrobial Resistance in Animals and the Environment; 2007 Dec 17–22; Tours, France.
- Hallin M, Denis O, Deplano A, de Mendonca R, De Ryck R, Rottiers S, Genetic relatedness between methicillin-susceptible and methicillin-resistant Staphylococcus aureus: results of a national survey. J Antimicrob Chemother. 2007;59:465–72. DOIPubMedGoogle Scholar
- Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43:5026–33. DOIPubMedGoogle Scholar
- Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Involvement of Panton-Valentine leukocidin–producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. DOIPubMedGoogle Scholar
- Lina G, Quaglia A, Reverdy ME, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–6.PubMedGoogle Scholar
- Vanhoof R, Godard C, Content J, Nyssen HJ, Hannecart-Pokorni E. Detection by polymerase chain reaction of genes encoding aminoglycoside-modifying enzymes in methicillin-resistant Staphylococcus aureus isolates of epidemic phage types. J Med Microbiol. 1994;41:282–90. DOIPubMedGoogle Scholar
- Ng LK, Martin I, Alfa M, Mulvey M. Multiplex PCR for the detection of tetracycline resistant genes. Mol Cell Probes. 2001;15:209–15. DOIPubMedGoogle Scholar
- Lewis HC, Molbak K, Reese C, Aarestrup FM, Selchau M, Sorum M, Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg Infect Dis. 2008;14:1383–9. DOIPubMedGoogle Scholar
- Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1–7. DOIPubMedGoogle Scholar
- van Rijen MM, van Keulen PH, Kluytmans JA. Increase in a Dutch hospital of methicillin-resistant Staphylococcus aureus related to animal farming. Clin Infect Dis. 2008;46:261–3. DOIPubMedGoogle Scholar
- Fanoy E, Helmhout LC, van der Vaart WL, Weijdema K, Santen-Verheuvel MG, Thijsen SF, An outbreak of non-typeable MRSA within a residential care facility. Euro Surveill [serial online]. 2009;14. Available from http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19080
- Wulf MW, Markestein A, van der Linden FT, Voss A, Klaassen C, Verduin CM. First outbreak of methicillin-resistant Staphylococcus aureus ST398 in a Dutch hospital, June 2007. Euro Surveill [serial online]. 2008;13. Available from http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8051
- Wulf MW, Sorum M, van Nes A, Skov R, Melchers WJ, Klaassen CH, Prevalence of methicillin-resistant Staphylococcus aureus among veterinarians: an international study. Clin Microbiol Infect. 2008;14:29–34. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 15, Number 7—July 2009
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Olivier Denis, Service de Microbiologie, Hôpital Erasme, 808 route de Lennik, 1070 Brussels, Belgium
Top