Volume 18, Number 9—September 2012
Letter
Rickettsia raoultii–like Bacteria in Dermacentor spp. Ticks, Tibet, China
To the Editor: Rickettsia raoultii is an obligate intracellular gram-negative bacterium belonging to the spotted fever group (SFG) of the genus Rickettsia. Genotypes RpA4, DnS14, and DnS28, originally isolated from ticks from Russia in 1999 (1), were designated as Rickettsia raoultii sp. nov. on the basis of phylogenetic analysis (2). R. raoultii has been found mainly in Dermacentor spp. ticks in several countries in Europe (3). It was detected in a Dermacentor marginatus tick from the scalp of a patient with tick-borne lymphadenitis in France (2), which suggests that it might be a zoonotic pathogen. We determined the prevalence of R. raoultii–like bacteria in Dermacentor spp. in highland regions in Tibet.
Ticks from sheep (Ovis aries) near Namuco Lake (a popular tourist destination 4,718 m above sea level) were collected and identified morphologically as D. everestianus and D. niveus ticks (4). Genomic DNA was extracted from individual specimens by using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). All DNA samples were amplified by using PCRs specific for the citrate synthase (gltA, 770 bp) gene (5) and the outer membrane protein A (ompA, 629 bp) gene (6). Some samples were amplified by using a PCR specific for the ompB (2,479 bp) gene (7).
Randomly selected amplicons for gltA (n = 27), ompA (n = 31), and ompB (n = 7) were cloned into the pGEM-T Easy vector (Promega, Shanghai, China) and subjected to bidirectional sequencing (Sangon Biotech, Shanghai, China). Sequences obtained were deposited in GenBank under accession nos. JQ792101–JQ792105, JQ792107, and JQ792108–JQ792166. Phylogenetic analysis was conducted for sequences we identified and sequences of recognized SFG rickettsial species available in Genbank by using the MegAlign program (DNASTAR, Inc., Madison, WI, USA) and MEGA 4.0 (8).
Of 874 tick specimens, 86 were D. everestianus ticks (13 male and 73 female), and 788 were D. niveus ticks (133 male and 655 female). Samples positive for gltA and ompA were considered SFG rickettsial species. Using this criterion, we found that 739 tick specimens (84.6%) were positive for Rickettsia spp. Of 86 D. everestianus ticks, 85 (98.8%) were positive for Rickettsia spp. and of 788 D. niveus ticks, 654 (83.0%) were positive. Infection rates for male and female D. niveus ticks were 87.9% and 82.1%, respectively. We found an overall prevalence of 84.6% for R. raoultii–like bacteria in Dermacentor spp. in the highland regions in Tibet.
Nucleotide sequence identities ranged from 99.2% to 100% (except for isolate WYG55, which had an identity of 98.6%) for the ompA gene and from 99.2% to 99.9% (except for isolate XG86, which had an identity of 98.5%) for the ompB gene. These results indicated that homology levels of most isolates were within species thresholds (ompA ≥98.8% and ompB ≥99.2%) (9). Isolate WYG55 showed the lowest identity (98.2%) among gltA gene sequences and the lowest identity (98.6%) among ompA gene sequences. Isolate XG86 showed lowest identity (98.5%) among ompB gene sequences. These results suggest that other Rickettsia spp. were among the investigated samples.
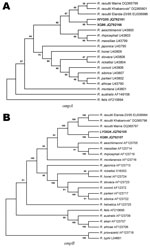
A BLASTn search (www.ncbi.nlm.nih.gov) for the obtained sequences was conducted. The best matches (highest identities) detected were with sequences of R. raoultii. However, comparison of our sequences with corresponding sequences of R. raoultii in GenBank showed identity ranging from 98.0% to 99.0% for ompA and from 98.1% to 99.0% for ompB, which did not meet the threshold (9) for R. raoultii. We compared the new sequences with corresponding reference sequences of universally recognized SFG group Rickettsia spp. in Genbank and constructed 2 phylogenetic trees (Figure). The new sequences were placed into separate branches, which were closely related to R. raoultii branches.
Prevalence of R. slovaca and R. raoultii was 6.5% and 4.5% in D. silvarum ticks in Xinjiang Uygur Autonomous Region of China (10). In contrast, we found that the overall prevalence of R. raoultii–like bacteria might be ≤84.6% in D. everestianus and D. niveus ticks in Dangxiong County in Tibet.
Our findings suggest that D. everestianus and D. niveus ticks are potential vectors of R. raoultii–like bacteria and indicate that spread of R. raoultii-like bacteria encompasses a large area in China. In the study sites, yak and Tibetan sheep are the major domestic animals, and rodents are the major wild animals. Rodents are also the major hosts of Dermacentor spp. ticks, which can transmit R. raoultii transstadially and transovarially (2). Animals bitten by infected ticks can acquire the pathogen and serve as natural reservoirs.
On the basis of phylogenetic analysis, we found that the Rickettsia spp. in ticks investigated represents a novel species, which can be designated Candidatus Rickettsia tibetani. However, additional phylogenetic studies are needed to obtain more information on the molecular biology of these bacteria.
Acknowledgments
We thank Robin B. Gasser for providing comments and revising the manuscript.
This study was supported by the 973 Program (2010CB530206), the Key Project of Gansu Province (1002NKDA035 and 0801NKDA033), the National Science Foundation of China ( 30800820, 30972182, 31072130, and 31001061), the 948 Program (2012-S04), the National Beef Cattle Industrial Technology System, Ministry of Agriculture (CARS-38), the Network for Excellence for Epizootic Disease Diagnosis and Control (FOOD-CT-2006-016236), and the Improvement of Current and Development of New Vaccines for Theileriosis and Babesiosis of Small Ruminants (PIROVAC) Project (KBBE-3-245145) of the European Commission, Brussels, Belgium.
References
- Rydkina E, Roux V, Rudakov N, Gafarova M, Tarasevich I, Raoult D. New Rickettsiae in ticks collected in territories of the former Soviet Union. Emerg Infect Dis. 1999;5:811–4. DOIPubMedGoogle Scholar
- Mediannikov O, Matsumoto K, Samoylenko I, Drancourt M, Roux V, Rydkina E, Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int J Syst Evol Microbiol. 2008;58:1635–9. DOIPubMedGoogle Scholar
- Spitalská E, Stefanidesova K, Kocianova E, Boldis V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp Appl Acarol. 2012;57:189–7. DOIPubMedGoogle Scholar
- Teng KF, Jiang ZJ. Economic insect fauna of China, Acari, Ixodidae. Beijing: Science Press; 1991.
- Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–61. DOIPubMedGoogle Scholar
- Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–65.PubMedGoogle Scholar
- Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 2004;42:4961–7. DOIPubMedGoogle Scholar
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. DOIPubMedGoogle Scholar
- Raoult D, Fournier PE, Eremeeva M, Graves S, Kelly PJ, Oteo JA, Naming of Rickettsiae and rickettsial diseases. Ann N Y Acad Sci. 2005;1063:1–12. DOIPubMedGoogle Scholar
- Tian ZC, Liu GY, Shen H, Xie JR, Luo J, Tian MY. First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China. Parasit Vectors. 2012;5:9. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 18, Number 9—September 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hong Yin, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Xujiaping 1, Lanzhou, Gansu, 730046, People’s Republic of China
Top