Volume 19, Number 11—November 2013
Research
Migration and Persistence of Human Influenza A Viruses, Vietnam, 2001–2008
Abstract
Understanding global influenza migration and persistence is crucial for vaccine strain selection. Using 240 new human influenza A virus whole genomes collected in Vietnam during 2001–2008, we looked for persistence patterns and migratory connections between Vietnam and other countries. We found that viruses in Vietnam migrate to and from China, Hong Kong, Taiwan, Cambodia, Japan, South Korea, and the United States. We attempted to reduce geographic bias by generating phylogenies subsampled at the year and country levels. However, migration events in these phylogenies were still driven by the presence or absence of sequence data, indicating that an epidemiologic study design that controls for prevalence is required for robust migration analysis. With whole-genome data, most migration events are not detectable from the phylogeny of the hemagglutinin segment alone, although general migratory relationships between Vietnam and other countries are visible in the hemagglutinin phylogeny. It is possible that virus lineages in Vietnam persisted for >1 year.
Understanding influenza dynamics in tropical regions is crucial for understanding global influenza epidemiology because dynamics between temperate and tropical regions are closely linked. Phylogenetic studies have supported eastern Asia, Southeast Asia, and the tropics as potential ecological sources of global influenza circulation (1,2), but others have suggested a variety of geographic regions as potential sources (3–5). Consequently, the role played by the tropics in the global epidemiology of influenza is still uncertain. Viral gene sequence data from tropical countries are crucial for understanding virus migratory routes within the tropics and between tropical and temperate countries.
Vietnam is an example of a tropical country that may play a major role in global influenza dynamics but for which relatively little is known about influenza epidemiology and genetic population structure of the viruses. Sentinel surveillance suggests that in Vietnam, influenza peaks 1–2 times per year, but neither the influenza-like illness (ILI) data nor the virologic confirmation data show a simple seasonal pattern; the trends for confirmed influenza cases fluctuate more than trends for ILI (6,7). Serologic studies indicate that annual influenza incidence in Vietnam is between 17% and 26% (8). The population of Vietnam is relatively young; according to contact patterns, most cases should occur among younger persons (9,10). Given Vietnam’s high population density and strong travel connections to eastern Asia, Southeast Asia, and Australia/New Zealand, Vietnam is as likely as any other country in eastern or Southeast Asia to support continuous, year-round circulation of a single influenza lineage (persistence) and potentially act as a global source of influenza viruses.
Previous global phylogenetic studies of influenza have demonstrated virus mixing globally (3,4,11), a lack of interseasonal persistence in temperate regions (1,2,11,12), and some evidence of persistence in subtropical regions (5,13). Time-series studies of confirmed influenza suggest (with exceptions [14]) that influenza does not exhibit the same strong and regular seasonality in tropical countries as it does in temperate zones (15–19) and that it could be constantly circulating throughout the year (20,21); however, in the latter 2 studies, phylogenetic analyses were not performed. We analyzed 240 newly sequenced influenza virus whole genomes from Vietnam, sampled through the Vietnam National Sentinel Surveillance System during 2001–2008 (6). We determined the relative strength of influenza migratory connections between Vietnam and the rest of the world, and we interpreted these results in the context of a sampling bias that seems to affect all sequence-based studies aiming for phylogeographic interpretations. On the basis of frequent sampling in 2007 and 2008, we assessed whether influenza viral lineages persisted in Vietnam during this period. However, we could not definitively conclude whether Vietnam represents a sink or a source population for influenza transmission.
Understanding global influenza migration and persistence patterns is crucial for maintaining a coordinated and efficient biannual strain selection process for influenza vaccine. Choices for future vaccine components will depend on recent availability of samples, and understanding each region’s contribution to global influenza circulation will help inform decisions based on viruses coming from highly connected or weakly connected regions.
Samples
During 2001–2008, as part of the Vietnam National Influenza Surveillance System, nasopharyngeal or throat swab samples were collected from patients seeking care for ILI at hospitals (6). Specimens were tested for influenza A and B viruses and were further subtyped for H1, H3, and H5 by reverse transcription PCR by using primers, probes, and reagents recommended by the Centers for Disease Control and Prevention and the World Health Organization (WHO). Samples that were positive for influenza A by PCR were selected for virus isolation, and isolates reaching a titer of 1:8 in hemagglutination assays were selected for sequencing analysis. All isolates were subtyped by using hemagglutination assays with reference antigens and antiserum from the WHO reagent kit. A total of 242 samples were shipped to the National Institutes of Health Influenza Genome Sequencing Project (USA) (22) for whole-genome sequencing at the J. Craig Venter Institute. Of the 242 samples, 2 were excluded from this analysis (1 that could not be sequenced and 1 from a patient with a mixed infection). The final dataset of the 240 whole-genome sequences comprised 145 influenza subtypes H3N2 and 95 H1N1 (GenBank accession nos. CY103972–CY105893). Table 1 shows the numbers and locations of the viruses.
Datasets
For phylogeographic analysis, we compiled influenza virus sequences of subtypes H1N1 and H3N2 into 2 datasets: a regional dataset of whole-genome sequences from Asia and Australia/New Zealand and a global dataset of geographically subsampled sequences (50 replicates) of the hemagglutinin (HA) segment (Technical Appendix). For subsampling, we randomly sampled 12 sequences per geographic region per year.
Phylogenetic Inference
Sequences were aligned by using the MUSCLE program, version 3.8 (23). Maximum-likelihood trees were inferred by using RAxML version 7.3.0 with 2,000 bootstrap replicates (24,25). For the regional HA datasets, phylogenetic trees with sampling date information were inferred by using BEAST version 1.6.2 (26) and a relaxed molecular clock (uncorrelated lognormal). The nucleotide substitution model was SRD06 + HKY85 + Γ, and the demographic models used were constant population size and Bayesian skyride (Technical Appendix).
Analysis of Regional Migration
Migration analysis was conducted by using a straightforward parsimony method in the PAUP* program (27,28). The 2,000 bootstrap trees and the best maximum-likelihood tree inferred by RAxML were read into PAUP*, and nucleotide sequences were replaced by single-letter location codes assigned to a set of predefined global regions (Technical Appendix Figures 5, 6). Changes in location code were mapped onto the branches of the trees by using an ACCTRAN parsimony criterion (28). Analysis was performed on all segments to determine whether migration histories differed among them, which could have been caused by reassortment among influenza virus RNA segments. A strong reassortment signal was verified when standard mosaic and phylogenetic criteria were used (29) (Technical Appendix Figure 2).
Analysis of Global Migration
For comparison, we performed the same migration analysis on the global dataset of 50 subsampled replicates for influenza virus subtypes H3N2 and H1N1. Migration matrices were built describing numbers of connections between 27 subtype H3N2 or 29 subtype H1N1 predefined geographic regions. We used Gephi software (30) to visualize the connections in the matrix. For regions with sufficient samples, we computed minimum distances to the trunk of the rooted phylogeny for all 50 subsampled trees (subtype H3N2 only) to determine whether viruses from different regions could be described as ancestral (close to the trunk) or derived (far from the trunk).
Regional Migration of Influenza Virus (H3N2) HA
The relationship between the subtype H3N2 HA sequences from Vietnam and other viruses sampled in the region is shown in Figure 1. Representative samples from Vietnam are available for 2003–2008 but not for 2006 when subtype H1N1 predominated. Viruses isolated in Vietnam show close relationships to viruses isolated in Hong Kong, Taiwan, Singapore, and Australia/New Zealand. Inferred from this tree were 20 parsimony-unambiguous migration events, 9 showing Vietnam–Hong Kong migration, 7 showing Vietnam–Australia/New Zealand migration, 2 showing Vietnam–Taiwan migration, 1 showing Vietnam–South Korea migration, and 1 showing Vietnam–Qatar migration. Clearly, because Hong Kong (68 sequences) and Australia/New Zealand (>500 sequences) were overrepresented in the regional dataset, most Vietnam migrations were associated with these 2 locations. Because we were initially uncertain how well a geographically regional phylogenetic tree would reflect the true migration patterns of influenza viruses in Vietnam, we performed a validation exercise to determine what proportion of global migration of influenza subtype H3N2 virus from Vietnam could be detected in a regional phylogenetic tree (Technical Appendix). Approximately 70% of Vietnam influenza migrations from a global analysis were also observed in the regional tree.
Regional Migration of Influenza Virus (H1N1) HA
The relationship between influenza subtype H1N1 HA sequences from Vietnam and other regional viruses is shown in Figure 2. Representative samples from Vietnam are available for 2001–2008 but not for 2004 and 2007. Inferred from this tree are 10 parsimony-unambiguous migration events, 6 showing Vietnam–Taiwan migration, 2 showing Vietnam–Japan migration, and 2 showing Vietnam–Australia/New Zealand migration. As in the analysis for subtype H3N2, these migration links correspond with the viruses that were sequenced from the region during 2001–2008.
Whole-Genome Migration Patterns
Because the regional trees included only sequences for which whole genomes were available, migration patterns were compared systematically across all 8 influenza segments. Because influenza viruses reassort, different event histories should be visible in phylogenies inferred separately for the 8 virus segments. Indeed, for the subtype H3N2 dataset, we observed a median of 14 parsimony-unambiguous migration events for the neuraminidase segment and a median of 41 for the matrix protein segment; the other segments fell somewhere in between (Table 2). Again, most migrations were with Australia/New Zealand and Hong Kong, indicating that the pattern of migration is similar across segments, although different numbers of migrations and different individual migration events are visible when different segments are analyzed. For the matrix protein and nonstructural protein segments of subtype H3N2 viruses, the large number of migration events may result from the larger number of topologically uncertain and polytomic nodes in these trees that had to be randomly resolved to compute the number of migration events; that is, sequences from one country could be mistakenly mixed with sequences from other countries, thus generating some artificial migration events in the parsimony analysis. The low confidence in the Vietnam–Singapore migration link for subtype H3N2 may result from the small number of whole-genome sequences available from Singapore, all of which were collected in 2003.
For subtype H1N1 viruses, we observed 6–16 migration events across the trees inferred for the 8 segments (Table 2). In the bootstrapped data, the Vietnam–Taiwan and Vietnam–Japan migratory connections seem to be approximately equal, despite the fact that the best maximum-likelihood tree showed 6 Vietnam–Taiwan connections and 2 Vietnam–Japan connections. The migratory connection between Vietnam and Australia/New Zealand seems to be somewhat weaker, possibly because of substantially less sampling of Australia/New Zealand viruses in the subtype H1N1 dataset. In the subtype H1N1 dataset, the number of migration events for the matrix protein and nonstructural protein segments did not increase.
Migration in Subsampled Global HA Trees
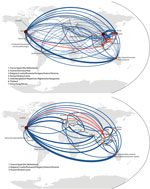
Because it is clear that the presence and number of samples from different regions influence migration analysis, migratory patterns were reanalyzed on the global subsampled dataset to reduce the geographic bias present from having higher numbers of samples available from some regions than others. Using global HA trees for subtype H3N2 and H1N1 sequences, we constructed full migration matrices including all parsimony-unambiguous migration events among our predefined regions (Technical Appendix Figures 5, 6). These migration networks are shown in Figure 3, where the United States is a major hub of influenza migration and eastern Asia and Australia/New Zealand play major roles. The subtype H3N2 data show Vietnam connected with most other countries in eastern and Southeast Asia, with the United States, and weakly with southern Asia. The subtype H1N1 data show Vietnam connected with the United States and Europe but weakly with other Asian countries. For both subtypes, the total number of migration events associated with each node in the network is correlated with the number of samples available for that node (all p values were <10−5; Kendall and Spearman tests). Note that sample numbers are not identical for each node because for some regions <12 sequences per year were available, and these regions did not need to be subsampled for those years. Hence, undersampling and oversampling can generate this correlation. Despite our attempt to reduce geographic bias in the global dataset, inference on migration events is still closely associated with regional availability of samples; this bias appears to affect all phylogeographic studies.
For H3N2 sequences, to determine whether any region has the characteristics of an ecological source, we computed the phylogenetic distance of sequences from 6 well-sampled regions (China, Hong Kong, Japan, Vietnam, Australia/New Zealand, and the United States) to the trunk of the global maximum-likelihood phylogenetic tree (Figure 4). In 2003, for example, across all 50 subsampled trees, sequences isolated in China were typically closest to the trunk of the phylogenetic tree, indicating that these sequences are ancestral to other viral sequences sampled in 2003; this finding is consistent with the global replacement of subtype H3N2 viruses by the A/Fujian/411/2002-lineage that occurred in 2003. In general, for the years 2003–2007, in no region were sequences consistently ancestral, indicating that it is unlikely that there is a single global source of human influenza viruses. The more likely global migration model involves periodic global strain replacements originating in different regions in different years (3,4). There were not sufficient samples from all regions/years to perform this analysis on the subtype H1N1 dataset.
Lineage Persistence
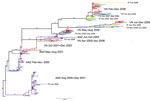
Figure 5 shows a Bayesian subtype H3N2 phylogenetic tree inferred from the time-stamped regional sequence data. The insets of this figure detail the 2007–2008 part of the phylogeny (87 sequences) and the coalescent times for the tips of these branches. It is difficult to draw a complete persistence picture for these viruses because of undersampling during the second quarter of 2007 and the first half of 2008 despite PCR-confirmed evidence of influenza virus activity during these periods (6). The median coalescent time for viruses from Vietnam sampled during this period is 37 days (interquartile range 21–72 days), and the insets in Figure 5 suggest that one of the lineages persisted in Vietnam for the 13 months from January 2007 through January 2008. An absence of samples from February through May 2008 makes it impossible to determine conclusively if this lineage persisted in Vietnam for the entire 2-year period.
To determine whether the lack of samples from other countries created an artifactual picture of lineage persistence in Vietnam during 2007–2008, we assembled a sequence set of all 672 viruses from Asia and Australia/New Zealand from 2006 through 2008. The maximum clade credibility tree of these sequences (Technical Appendix Figure 4) indicates that the Vietnam lineages separate into >10 distinct lineages when viewed in the context of all Asian/ Australia/New Zealand influenza viruses. One of these lineages persisted in Vietnam for 15 months (Technical Appendix Figure 1 panel A), and another persisted for 10–12 months (Technical Appendix Figure 1, panel B), suggesting that lineage persistence of ≥1 year may have occurred in Vietnam during 2007–2008. However, this type of analysis is very sensitive to phylogenetic uncertainty because the individual lineages (or subclades) contain few sequences and may not be robust to small changes in tree topology.
According to our analysis, the major migratory routes of influenza virus pass through the United States, eastern Asia, and Australia/New Zealand. Europe—despite its population density and consistency of wintertime influenza epidemics—was slightly less connected to other parts of the world when compared with the United States. These results are consistent with those of previous studies that showed eastern Asia (2) and tropical Asia (1) as key influenza source populations and the United States as a major contributing region (3). The new sequence data in this analysis support strong migratory connections between Vietnam and neighboring countries, the United States, and Europe. Our regional phylogenetic analysis supports a strong connection between Vietnam and Australia/New Zealand, but the global analysis reveals that Australia/New Zealand sequences are more closely related to sequences from Asian countries other than Vietnam. In addition, the inferred phylogenies provide evidence of virus persistence in Vietnam for >1 year. This is a major finding because strong migratory links and persistence are the 2 key features for a proposed source region for influenza transmission; long-term persistence in tropical regions may be associated with more antigenic evolution and immune escape if it can be shown that longer persistence gives the virus population more time to accumulate and fix antigenic changes (2,31,32).
In general, persistence analyses are difficult even with regular sequence sampling and weekly virologic confirmations. When attempting to assess the likelihood of influenza persistence in a focal region (e.g., Vietnam), we must sample outside the focal region to determine whether local viruses have been reintroduced from elsewhere. However, the more sampling in the nonfocal region, the more likely it becomes that we sample nonfocal viruses similar to focal viruses and that more diversity is detected in the nonfocal region, making it seem basal (closer to the root) to the focal region. There are no clear criteria for whether we have undersampled or oversampled the focal or the nonfocal region; thus, it is extremely difficult to state with certainty that an apparently local lineage has persisted in the same location. For the 2007–2008 Vietnam influenza sequences, viruses were sampled for most of this period and coalescence times were generally short, indicating that most of these viruses have a relatively recent ancestor in Vietnam. These data are consistent with and provide evidence for lineage persistence in Vietnam during this time. However, we know of no unbiased test that can reject the possibility of virus introduction. The perfect dataset for demonstrating lineage persistence would seem to be 52 viruses sampled in 52 weeks, with consecutive viruses differing at 0 or 1 nt positions.
A major limitation of all migration analyses performed with sequence data is geographic sampling bias: undersampling and oversampling. The more sequences that are available for a given location, the more likely it is that 1 of these sequences will be a recent immigrant, identifiable by the presence of similar sequences from other locations. To overcome this bias, subsampling is typically conducted (3,5) to ensure that the same numbers of sequences are used from each region. In the situation when too few sequences are available from a particular location, a smaller number of migratory links will be able to be inferred for that location. This second bias cannot be corrected with a subsampling strategy.
Our analysis of the global subsampled dataset showed that sample counts and strength of migratory connections were highly correlated. It has so far been impossible to determine the causal direction in this correlation. A migration signal can be weak because of a dearth of samples. Conversely, the small number of samples can be the result of low influenza activity and a corresponding weak migratory connection with other regions. The directionality of causation cannot be determined from sequence data alone. A sequence sampling strategy must be devised in the context of an influenza surveillance system, and the epidemiologic data and sequence data must be analyzed jointly. Disease prevalence and sequence data should be directly linked to provide a denominator to help determine whether undersampling or oversampling are truly occurring, which would allow for correction of sampling numbers across regions.
Despite this seemingly obvious point about oversampling, the counterpoint is that oversampling in influenza sequence data occurs with a high degree of pseudoreplication. Influenza sequence sampling in most scientific studies and public health contexts is conducted in such a way that each additional sequence sample is not an independent observation but, rather, is an observation with a high degree of correlation to recently collected samples (33). These pseudoreplicated samples should not, in principle, generate additional artificial migration events into the analysis because the dependency structure of the samples is entirely accounted for in the phylogeny. Nevertheless, a correlation between sample number and migration strength persists in the data, partially, at least, because a larger number of samples increases the probability that a distant recently introduced lineage is sampled.
New approaches are needed in order to fully account for all spatial, evolutionary, and epidemiologic dependencies in phylogeographic analyses. For recent phylogeographic studies, Bayesian approaches have been the method of choice (1,3,4,34–37), primarily because of their ability to account for uncertainty in evolutionary, demographic, and migratory parameters, but especially because of their ability to incorporate topological uncertainty into phylogenetic analyses. If these methods can be further developed to incorporate representativeness uncertainty—essentially, a prior distribution on the size of the sampling pool to account for the fact that some parts of the phylogeny will be oversampled while others will be undersampled—then this type of Bayesian analysis could serve as a powerful auxiliary tool in phylogeography, enabling us to determine whether sampling bias has a larger effect in some regions than others. Another role for Bayesian analysis of influenza sequences will be the application of Bayesian phylogeographic methods on whole-genome sequence data (1). For highly reassortant datasets, the presence of independent migration signals in 8 phylogenies (for the influenza virus 8 RNA segments) should act to reduce uncertainty for the inferred migration parameters.
We intended to elucidate the migratory pathways of influenza into and out of Vietnam and the likelihood of virus persistence in Vietnam. For each of these objectives, we recommend that future studies link phylogenetic analysis with prevalence data, allowing for correction of known biases and providing crucial complementary epidemiologic evidence for migration and persistence. If the source–sink framework is an oversimplification of global influenza circulation (3–5), Vietnam probably plays both roles on different occasions, given its close connections to other countries in Asia, Europe, and the United States.
Dr Le is head of the Virology Department at the Vietnam National Institute for Hygiene and Epidemiology, Hanoi, Vietnam. Her research interests are virology, public health, and human and avian influenza viruses.
Acknowledgments
We thank WHO Vietnam, the US Centers for Disease Control and Prevention, H. Suzuki, and R. Saito for helping establish influenza sentinel surveillance in Vietnam. Thanks to E.C. Holmes for critical review of this manuscript.
H.M.L., P.H., M.F.B. are supported by the Wellcome Trust (089276/B/09/7, 098511/Z/12/Z, WT/093724). T.T.L. is supported by a Royal Society Newton International Fellowship (UK). Computing and hardware costs were funded by the Li Ka Shing, University of Oxford Global Health Programme (LG05). This project has been funded in part with federal funds from the Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, under contract no. HHSN272200900007C.
References
- Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453:615–9. DOIPubMedGoogle Scholar
- Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320:340–6. DOIPubMedGoogle Scholar
- Bedford T, Cobey S, Beerli P, Pascual M. Global migration dynamics underlie evolution and persistence of human influenza A (H3N2). PLoS Pathog. 2010;6:e1000918. DOIPubMedGoogle Scholar
- Bahl J, Nelson MI, Chan KH, Chen R, Vijaykrishna D, Halpin RA, Temporally structured metapopulation dynamics and persistence of influenza A H3N2 virus in humans. Proc Natl Acad Sci U S A. 2011;108:19359–64 and. DOIPubMedGoogle Scholar
- Cheng X, Tan Y, He M, Lam TT-Y, Lu X, Viboud C, Epidemiological dynamics and phylogeography of influenza virus in southern China. J Infect Dis. 2013;207:106–14. DOIPubMedGoogle Scholar
- Nguyen HT, Dharan NJ, Le MTQ, Nguyen NB, Nguyen CT, Hoang DV, National influenza surveillance in Vietnam 2006–2007. Vaccine. 2009;28:398–402. DOIPubMedGoogle Scholar
- Oxford University Clinical Research Unit. Influenza-like illness (ILI) in Ho Chi Minh City. 2013 [cited 2013 Mar 4]. www.ili.vn.
- Horby P, Mai Le Q, Fox A, Thai PQ, Thi Thu Yen N. Thanh le T, et al. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: The Ha Nam Household Cohort Study I. Am J Epidemiol. 2012;175:1062–74.
- Boni MF, Manh BH, Thai PQ, Farrar J, Hien TT, Hien NT, Modelling the progression of pandemic influenza A(H1N1) and the opportunities for reassortment with other influenza viruses. BMC Med. 2009;7:43. DOIPubMedGoogle Scholar
- Horby P, Thai PQ, Hens N, Yen NTT, Mai LQ, Thoang DD, Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS ONE. 2011;6:e16965. DOIPubMedGoogle Scholar
- Nelson MI, Simonsen L, Viboud C, Miller MA, Holmes EC. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PLoS Pathog. 2007;3:1220–8. DOIPubMedGoogle Scholar
- Nelson MI, Simonsen L, Viboud C, Miller MA, Taylor J, St. George K, Stochastic processes are key determinants of short-term evolution in influenza A virus. PLoS Pathog. 2006;2:e125. DOIPubMedGoogle Scholar
- Tang JW, Ngai KLK, Lam WY, Chan PKS. Seasonality of influenza A (H3N2) virus: a Hong Kong perspective (1997–2006). PLoS ONE. 2008;3:e2768. DOIPubMedGoogle Scholar
- Doraisingham S, Goh KT, Ling AE, Yu M. Influenza surveillance in Singapore: 1972–86. Bull World Health Organ. 1988;66:57–63 .PubMedGoogle Scholar
- Simmerman JM, Uyeki TM. The burden of influenza in East and South-East Asia: a review of the English language literature. Influenza Other Respi Viruses. 2008;2:81–92. DOIPubMedGoogle Scholar
- Wong CM, Yang L, Chan KP, Leung GM, Chan KH, Guan Y, Influenza-associated hospitalization in a subtropical city. PLoS Med. 2006;3:e121. DOIPubMedGoogle Scholar
- Yap FHY, Ho P-L, Lam K-F, Chan PKS, Cheng Y-H, Peiris JSM. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73:617–23. DOIPubMedGoogle Scholar
- Shek LPC, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4:105–11. DOIPubMedGoogle Scholar
- Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS ONE. 2007;2:e1296. DOIPubMedGoogle Scholar
- Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. DOIPubMedGoogle Scholar
- Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007;165:1434–42. DOIPubMedGoogle Scholar
- Ghedin E, Sengamalay NA, Shumway M, Zaborsky J, Feldblyum T, Subbu V, Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature. 2005;437:1162–6. DOIPubMedGoogle Scholar
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7 . DOIPubMedGoogle Scholar
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008;57:758–71. DOIPubMedGoogle Scholar
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–90. DOIPubMedGoogle Scholar
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. DOIPubMedGoogle Scholar
- Swofford DL, Maddison WP. Reconstructing ancestral character states under Wagner parsimony. Math Biosci. 1987;87:199–229. DOIGoogle Scholar
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland (MA): Sinauer Associates; 2002.
- Boni MF, de Jong MD, van Doorn HR, Holmes EC. Guidelines for identifying homologous recombination events in influenza A virus. PLoS ONE. 2010;5:e10434. DOIPubMedGoogle Scholar
- Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. Third International AAAI Conference on Weblogs and Social Media; 2009 May 17– 20; San Jose, California [cited 2013 Mar 4]. http://www.aaai.org/Library/ICWSM/icwsm09contents.php
- Boni MF, Gog JR, Andreasen V, Feldman MW. Epidemic dynamics and antigenic evolution in a single season of influenza A. Proc Biol Sci. 2006;273:1307–16. DOIPubMedGoogle Scholar
- Adams B, McHardy AC. The impact of seasonal and year-round transmission regimes on the evolution of influenza A virus. Proc Biol Sci. 2011;278:2249–56. DOIPubMedGoogle Scholar
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford (UK): Oxford University Press; 1991.
- Pybus OG, Suchard MA, Lemey P, Bernardin FJ, Rambaut A, Crawford FW, Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc Natl Acad Sci U S A. 2012;109:15066–71. DOIPubMedGoogle Scholar
- Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLOS Comput Biol. 2009;5:e1000520 . DOIPubMedGoogle Scholar
- Rabaa MA, Hang VTT, Wills B, Farrar J, Simmons CP, Holmes EC. Phylogeography of recently emerged DENV-2 in southern Viet Nam. PLoS Negl Trop Dis. 2010;4:e766 . DOIPubMedGoogle Scholar
- Raghwani J, Rambaut A, Holmes EC, Hang VT, Hien TT, Farrar J, Endemic dengue associated with the co-circulation of multiple viral lineages and localized density-dependent transmission. PLoS Pathog. 2011;7:e1002064 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 11—November 2013
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Maciej F. Boni, Oxford University Clinical Research Unit, Hospital for Tropical Diseases, 764 Vo Van Kiet St, Q5, Ho Chi Minh City, Vietnam
Top