Volume 19, Number 11—November 2013
Research
Common Epidemiology of Rickettsia felis Infection and Malaria, Africa
Abstract
This study aimed to compare the epidemiology of Rickettsia felis infection and malaria in France, North Africa, and sub-Saharan Africa and to identify a common vector. Blood specimens from 3,122 febrile patients and from 500 nonfebrile persons were analyzed for R. felis and Plasmodium spp. We observed a significant linear trend (p<0.0001) of increasing risk for R. felis infection. The risks were lowest in France, Tunisia, and Algeria (1%), and highest in rural Senegal (15%). Co-infections with R. felis and Plasmodium spp. and occurrences of R. felis relapses or reinfections were identified. This study demonstrates a correlation between malaria and R. felis infection regarding geographic distribution, seasonality, asymptomatic infections, and a potential vector. R. felis infection should be suspected in these geographical areas where malaria is endemic. Doxycycline chemoprophylaxis against malaria in travelers to sub-Saharan Africa also protects against rickettsioses; thus, empirical treatment strategies for febrile illness for travelers and residents in sub-Saharan Africa may require reevaluation.
Investigations examining the etiologic spectrum of fever of unknown origin in Africa rapidly progressed during 2008–2011 (1–3), providing increased knowledge about bacterial infections. Bacterial agents that have been most frequently identified in North and sub-Saharan Africa by culture are non-typhoidal Salmonella, Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, and Mycobacterium tuberculosis (2). Several studies have assessed the effect of fastidious bacterial infections in systemic febrile illness, including Rickettsia felis (4–6), Coxiella burnetii (7), Tropheryma whipplei (3), and Borrelia spp. (1,8). Tourism, immigration, international business travel, international aid work, and the deployment of troops overseas were documented as contributors to a tremendous increase in international travel during 1996–2004 (9). International tourist arrivals reached 940 million worldwide during 2010, an increase of 6.6% over 2009, and the current total number of international migrants has increased to an estimated 214 million persons in 2012 (10). Consequently, physicians in the Western hemisphere increasingly encounter febrile patients returning from international travel who were exposed to tropical infections that the physicians are unfamiliar with (9,10). Among international travelers, malaria, dengue, and rickettsiosis are among the most identified etiologies of febrile illness, and exposure to mosquitoes is reported as the most common source of fever (11).
Rickettsia felis, an obligate intracellular Gram-negative bacterium belonging to the spotted fever group of Rickettsia, has been shown to be a common agent of bloodstream infections in among humans Senegal and Kenya, identified in 7% of the population evaluated (4–6). However, the epidemiology (including vectors and reservoirs) and clinical picture of this emerging infection in the rest of Africa is largely unknown (12,13). During 2011, a possibly primary infection with R. felis, named “yaaf,” was hypothesized in the case of an 8-month-old girl in Senegal with polymorphous skin lesions (12).
The considerable frequency of R. felis infections observed in febrile patients in malaria-endemic regions and the many relapses previously reported (4,5) led us to investigate the possible correlation of R. felis and that of the parasite, Plasmodium falciparum, a known vector of malaria. The reservoirs for malaria and many rickettsial species are mammals, including humans; humans have long been known to be a reservoir for malaria, and were documented as the reservoir for R. prowazekii, the agent of epidemic typhus (14). Vectors for both organisms are arthropods: for rickettsial diseases vectors are typically ticks, lice or mites, and infected humans are susceptible to relapse (such as epidemic and scrub typhus) (14).
The vectors for malaria are mosquitoes of the genus Anopheles that breed in warm and humid areas (15). Malaria is particularly common among young patients, because progressive immunity develops following multiple infections as the child grows older. Great apes in Cameroon were recently identified as targets or possibly the origin of malaria (16). R. felis has recently been detected in Anopheles gambiae mosquitoes in molecular form S, in Aedes albopictus mosquitoes, and in gorilla fecal samples (17–19). These elements suggest comparable features within the epidemiologic cycles of malaria and R. felis infection. In addition, co-infections by R. felis and P. falciparum have been reported in Kenya (5). To prove the hypothesis of the similar epidemiology of malaria and R. felis infection, target populations, clinical phenomena (relapses and bacteremia in apparently asymptomatic patients), and geographic and seasonal distribution should be compared. The objective of this work is to clarify the epidemiology of R. felis infection and to compare it with malarial epidemiology.
Study Areas and Participants
Febrile Patients
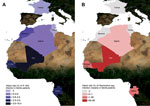
During June 2010‒March 2012, a cohort of 2,075 patients (67% <15 years of age; sex ratio, 1:1) from 14 health centers distributed throughout rural Senegal (Senegal study sites S1-S6) were enrolled in this study. The study sites spanned various ecosystems, from dry regions in the north (Dielmo, Senegal study region 1, S1, Ndiop-S2, Keur Momar Sarr–S3, and Niakhar-S4) to humid regions in the south (Basse-Casamance-S5 and Kedougou-S6) that had a rainy season during June through October (Technical Appendix Table). In addition, patients from various medical facilities were included: 100 from rural Mali dispensaries: Diankabou-Mali study site M1 and Kole-Mali study site M2; 50 from Franceville, in urban Gabon (pediatric consultation); 183 from Sfax, Tunisia (infectious diseases and pediatric departments); 266 from Oran, Algeria (department of infectious diseases); 48 from the Kenitra region, rural Morocco (dispensaries); and 400 from Marseille, France (hospital emergency units) (Figure 1). Questionnaires and informed consent forms were completed upon enrollment in the study. For each febrile patient (axillary temperature >37.5°C), an interview was conducted, a blood sample (200 µl blood containing EDTA) was collected, and a medical examination was performed. The national ethics committees of Senegal, Gabon, and France approved this project (No. 0–00.87MSP/DS/CNERS and No. 001380MSP/DS/CNERS).
Control Group
Samples were obtained from 400 afebrile persons (62% >15 years of age) from S1–2 who participated in a longitudinal study of malaria (20) and 100 persons from France who were under the medical care of 1 of the authors (D.R.) for conditions other than malaria.
Arthropod Collection in Senegal
Arthropod specimens collected in Senegal consisted of 949 adult mosquitoes from 3 locations (Table 1, 154 mosquito larvae from Mariste, Dakar, 370 ticks from 2 locations, 160 adult bed bugs from 6 locations, and 384 midges from 2 locations. The Anopheles arabiensis mosquito larvae were collected from breeding sites in Mariste, Dakar. The pooled larvae were maintained under laboratory conditions until they grew to the adult stage. In sites S1–2, 144 adult ticks (2 Rhipicephalus spp., 4 Argas persicus, and 138 Ornithodoros sonrai) from 55 burrows inside of 16 human dwellings were collected. A total of 226 Ornithodoros capensis ticks were manually collected from the nests of great cormorants (Phalacrocorax carbo) in Sarpan Island (îles de la Madeleine) near Dakar. Bed bugs were manually captured from the beds of ill persons. The collection of Culicoides spp. was performed in S1–2 by using overnight posed CDC light traps with 0.7-mm mesh size. The arthropods were identified at the species level by using morphological characteristics according to identification keys.
Molecular Analysis
DNA was extracted by using the 2-stage protocol for a QIAamp kit (QIAGEN, Hilden, Germany) for the S4–6 groups (3,4,7), and a Biorobot EZ1 Workstation (QIAGEN, Courtaboeuf, France) was used to extract DNA from samples from S1–2, Algeria, Tunisia, Morocco, and France. In Gabon, the DNA Blood Omega Bio-tek-E.Z.N.A method (Omega Bio-tek, Norcross, GA, USA) was used according to the manufacturer’s protocol. For all locations, DNA was eluted in 100 µL of elution buffer, and 5 µL was used per reaction.
Quantitative real-time reverse transcription PCR (qRT-PCR) was performed by using a 7900HT-thermocycler (Applied Biosystems) with the QuantiTect-Probe PCR Kit (QIAGEN, Courtabeuf, France). Only samples positive for the β-actin gene product were considered reliable (3); thus, 51 and 9 samples from Senegal and Algeria, respectively, were excluded. All samples were screened by using a Rickettsia genus-specific qRT-PCR targeting the gltA gene and an R. felis-specific qRT-PCR targeting the bioB gene (4). The positive samples were tested by a second R. felis-specific qRT-PCR targeting the orfB gene (18). A sample was considered positive when the qRT-PCRs were positive for the 2 different specific genes. Positive samples from arthropods were further tested for plasmid pRFδ (21) and by a newly designed R. felis-specific qRT-PCR targeting the vapB1 gene with the primers VapB1.R (5′-AGGCGAAAGCTTTGACGTG-3′) and VapB1.F (5′-TGTCTTTCATGAATTGATCAGCA-3′) and the probe VapB1.P (6-FAM-5′-AAGGCTTGGTTTCTGCGGGC-3′TAMRA).
Blood smears stained with Giemsa were examined for the samples collected in Gabon. All other samples were tested by using a Plasmodium-genus specific qRT-PCR targeting the Cox-1 gene found in all Plasmodium species; the primers Psp_15.F (5′-AGGAACTCGACTGGCCTACA-3′) and Psp_16.R (5′-CCAGCGACAGCGGTTATACT-3′) and the (6FAM-5′-CGAACGCTTTTAACGCCTGACATGG-3′TAMRA) probe were used. The positive samples were subsequently tested by Plasmodium-genus specific qRT-PCR targeting 18S rRNA with the primers Plasmo_18S_2_MBF (5′-AGGCAACAACAGGTCTGTGA-3′) and Plasmo_18S_2_MBR (5′-GCAATAATCTATCCCCATCACG-3′) and the (6FAM-5′- GAACTAGGCTGCACGCGTGCTACA-TAMRA-3′) probe.
Statistical Analysis
Statistical analyses were performed by using the Statcalc module of Epi Info 3.5.3 (Centers for Disease Control and Prevention, Atlanta, GA, USA) to calculate the χ2 values for the incidence rate trends calculated for each country. PASW Statistics software 17.0 (IBM, SPSS Inc., Armonk, NY, USA) was used to perform Pearson correlation analyses. The relative risk (RR) and the 95% CI of the risk were calculated by using either the Mantel-Haenszel χ2 test or Fisher’s exact test. The statistical significance of the χ2 values was evaluated at α = 0.05. The attack rates of R. felis infection and malaria were calculated for each country, site, sex, and age range. In contrast, the incidence rates of R. felis infection and malaria for S1–2 were calculated monthly and yearly from June 13, 2010 through October 13, 2011. The data from a study performed in 2009 (4) were combined with those of this study to determine the frequency of relapses or re-infections of R. felis infections in S1–2.
Rickettsia felis Detection
Senegal
The attack rate of R. felis infections in febrile patients was 15% (312/2,024); those infections occurred primarily during the rainy season rather than the dry season (207/1,105 vs. 105/916, respectively; p<0.0001). The risk of developing R. felis infection was 1.6× higher during the rainy period (95% CI 1.3–2) than during the dry period. When calculated by site, substantial differences in the rates of R. felis infection were observed (Table 2). The highest attack rates were observed in S5–6, reaching 40% (92/231) from August‒October 2011. The lowest attack rate was observed in S1–2 (7%–8%) and was significantly lower than that observed at the 4 other sites S3–6 (p≤0.001) (Table 2).
Incidence rates were obtained from 2 health centers (Figure 2). In 2011, the incidence rate of R. felis in S1 was 6.7 (4.8‒9.0) per 100 person-years or 0.55 (0.39‒0.76) per 100 person-months; the incidence rate in S2 was 3.1 (1.8‒4.9) per 100 person-years or 0.26 (0.15‒0.41) per 100 person-months during the same period. In S1–2, a significant difference was found between the incidence of R. felis for patients <15 years of age, which was 0.23 (0.16‒0.31) per 100 person-months, and the incidence in patients >15 years of age, which was 0.10 (0.06‒0.15) per 100 person-months (relative risk [RR] 2.38, 95% CI 1.34–4.28, p = 0.003). When the incidence rates by age group were calculated according to sex, a significant difference was observed only in the male group, in which the incidence rate was significantly higher in the patients <15 years of age than in the patients >15 years of age (0.29 vs. 0.07 per 100 person-months, RR 5.97, 95% CI 2.28–17.15, p = 0.001).
Table 3 shows the age distribution of R. felis infection. The occurrence of R. felis infection was significantly lower in patients 1–3 years of age (10%) than in patients >4 years of age (p = 0.03 for patients 4‒6 years of age (15%); p = 0.003 for patients 7‒15 years of age (16%); p = 0.004 for patients 16 to 29 years of age (16%); p = 0.002 for those >30 years of age (17%). The sex ratio for R. felis was 145M/162F (1:1.1). No deaths associated with R. felis infection were registered.
Combining these data with our preliminary report of 8 infected patients during 2008–2009 in S1–2 (4), we identified 61 patients with R. felis infections among a total of 456 villagers tested in S1–2. A second R. felis infection was diagnosed in 5 patients after 44 to 911 days, and 1 patient was positive for R. felis infection a second and third time at days 378 and 441, respectively. The 6 patients (4 male, 2 female) who had relapses or re-infections were from S1, and 5 were <6 years of age.
Other Countries
Samples from 3 patients (3%, 3/100) in rural Mali (M1, 1/50; M2, 2/50), 5 patients (10%, 5/50) in urban Gabon, 1 patient (2%, 1/48) in rural Morocco, and 2 patients (1%, 2/257) in Algeria were positive for R. felis (Table 2). Conversely, R. felis DNA was not detected among the samples from febrile persons in France and Tunisia.
When the R. felis infection rates of the different countries were compared, R. felis was detected more often in countries with high malaria rates compared with countries with low malaria rates (Senegal, Gabon, and Mali vs. Algeria, Tunisia, Morocco, and France; p<0.001) (Figure 1). The trend analysis showed a significant linear trend of increasing risk for R. felis infection; a lower risk was shown in northern countries (France, Tunisia, Morocco, and Algeria) and a higher risk in southern countries (Mali, Gabon, and Senegal) (p < 0.0001). The probability of R. felis infection was 1.00 for Algeria (baseline), 2.8 for Morocco, 4 for Mali, 14.5 for Gabon, and 24 for Senegal.
Malaria
Senegal
The attack rate of Plasmodium spp. in febrile persons from Senegal was 21% (400/1868, 206 females); those infections occurred significantly more often during the rainy season compared with the dry season (256/1042 vs. 144/822, respectively; p = 0.0002). The risk for malaria was 1.4× higher during the rainy period than during the dry period (95% CI 1.2–1.7, p<0.0001). The highest rate was in southeastern S6, whereas the lowest rate, 11% (37/350), was in southwestern S5 (Table 2). During the same time period, the incidence rate of malaria was 17.6 per 100 person-years or 1.46 per 100 person-months for S1 and 5.1 per 100 person-years or 0.42 per 100 person-months for S2. The highest incidence of malaria was among patients <15 years of age in S1–2 (0.55 (0.44–0.67) versus 0.22 (0.16–0.30) per 100 person-months, RR 2.51, 95% CI 1.73–3.65, p<0.0001).
When the incidence rate by age group was calculated according to sex, the highest incidence was found among girls <15 years of age: 0.47 (0.37–0.65) versus 0.25 (0.16–0.37) per 100 person-months, RR 1.92, 95% CI 1.15–3.18, p = 0.01; and boys (0.62 (0.47–0.81) versus 0.18 (0.10–0.30) per 100 person-months, RR 3.38, 95% CI 1.90–5.99, p<0.0001) groups. Table 3 shows the age distribution of malaria. Patients 7‒15 years of age (33%) were infected with Plasmodium spp. significantly more often than those in other age groups (15% for patients 1‒3 years of age, p<0.0001 to 23% for patients 4‒6 years of age, p = 0.004). Co-infection of Plasmodium spp. and R. felis was found in 66 case-patients (23%, 66/285), mostly in women (61%) and in children 7‒15 years of age (43%).
Other Countries
Plasmodium DNA was detected in 90% of the blood samples collected in Mali; 3 patients with malaria from Mali were co-infected with R. felis (Table 2). In Gabon, samples from 38% (19/50) of the patients tested positive for malaria by using blood smears; 2 of those patients were co-infected with R. felis. We most likely misdiagnosed malaria among the patients in Gabon, as based on the lower sensitivity and high specificity of microscopy versus PCR as the standard (22). Plasmodium DNA was not detected in the samples from Tunisia, Morocco, or France. However, 1 Plasmodium spp.-positive sample was collected in Algeria from a 21-year-old woman who was hospitalized for high fever, chills, and sweats after having spent >2 months visiting her family in Niger without malaria chemoprophylaxis.
Correlation of R. felis with Malaria
Using the Pearson correlation test, we found a significant correlation between the number of patients infected with R. felis and those infected with Plasmodium spp. (p<0.002): a higher number of R. felis infections correlated with a higher number of malaria cases. A significant correlation was also found for seasonality for infection by both pathogens: most cases occurred during the rainy period (p<0.0001). In addition, children <3 years of age were infected with both organisms less often than persons >4 years of age, and the Pearson test showed a significant correlation between R. felis and malaria (p = 0.001) for this age group.
Control Group
R. felis DNA was detected in 4% of the afebrile persons (17/391) from Senegal, 12 of whom were children (<15 years of age); malaria was detected in 5 afebrile persons, 3 of whom were children. Both pathogens were detected significantly less often in afebrile patients than in febrile patients (p<0.001). DNA from R. felis and Plasmodium spp. were not detected among persons in the control group in France.
Arthropod Study
Samples from 9 mosquitoes (≈1%, 9/1,103) and 1 bed bug (≈1%, 1/160) tested positive in 2 R. felis-specific qRT-PCRs (Tables 1,4). The pRFδ plasmid was detected in 8 mosquito samples (21). In Dakar, 1% (2/154) of the An. arabiensis mosquitoes collected were positive for R. felis, including 1 male, suggesting transovarian transmission. One Aedes luteocephalus from Ferlo 0.5% (1/203) was positive for R. felis. In S1, 15% (6/40) mosquitoes collected in September 2012 were positive for R. felis, including 1 An. ziemanni, 1 An. pharoensis, 2 Mansonia uniformis, and 2 An. funestus. None of the 24 mosquito samples collected from this region in July tested positive. In addition, 1 Cimex hemipterus bed bug (3%) (1/39), collected from a household in S1 in February 2012, tested positive. No R. felis DNA was detected in soft or hard ticks or in Culicoides species.
This study shows that Rickettsia felis is an emerging pathogen commonly detected in sub-Saharan rural Africa. We are confident that our molecular results are reliable and that the negative results in samples from France illustrate a correlation between R. felis infection and malaria with regard to the geographic distribution and seasonality. A trend of higher risk for R. felis infection in southern countries than in northern countries was revealed; the highest risk for R. felis infection was in rural Senegal (24 times than in Algeria). In Senegal, DNA from Plasmodium spp. and R. felis were detected at high levels, mostly during the rainy season and among children <15 years of age (Figure 2), but no coincidental relationship was found. The incidence of co-infection of R. felis and malaria was lower in Senegal (23%) than in Kenya (79%) (5), but higher than the rate of simultaneous bacterial bloodstream infections and malaria parasitemia, which ranged from 6% in rural Mozambique (23) to 11% in Nairobi (24). Mixed infections for rickettsioses, including co-infections with malaria or with other bacteria (Leptospira spp., Coxiella burnetii, and Burkholderia pseudomallei) have been described (25).
R. felis was detected in afebrile persons, most of whom were children <15 years of age, confirming the previously reported results in Kenya (5). Although rickettsioses have not previously been reported in afebrile persons, low-grade Plasmodium parasitemia has been reported among persons without a fever (26). This result should be confirmed by culture, but R. felis has never been isolated, even from acutely ill patients. Nonetheless, the absence of positive tests in the control group located in France confirmed the specificity of our tests. The S1–2 population was screened serologically for R. felis, and low titers were identified in 1 of 479 serum samples tested (27), which is substantially lower than the seroprevalence of other spotted fever group rickettsiae. The mechanism of absence of a serologic response and the occurrence of multiple re-infections or relapses of R. felis should be investigated further.
In this work, we demonstrated a greater frequency of R. felis during the rainy season among children in the subtropical zones, a period coinciding with circulation of P. falciparum. There are other seasonal diseases, including influenza, which are most common during the rainy season in subtropical Africa, particularly in Senegal (28). Influenza is a disease found throughout the year, with seasonal peaks, in Africa; none of the tested patients had influenza symptoms. Furthermore, leptospirosis, for which rickettsial disease could be mistaken, has not been documented in Senegal. Last, the most common seasonal disease in the most northern part of the intertropical area is malaria; a disease, however, which is common in all seasons in equatorial wetlands. These data, for which confirmation is needed, show a seasonal correlation between R. felis and malaria; the correlation is related to the presence and activity of Anopheles mosquitoes. Although the cat flea, Ctenocephalides felis, is currently the only known vector of R. felis, a variety of other arthropods have been suspected, including different flea species, ticks, mites, and lice (13). In Senegal, the source of R. felis is yet to be determined. We did not detect R. felis in fleas that were screened during 1 year in S1 and S2 (13). In other studies, R. felis was not detected in soft or hard ticks (27,29), tsetse flies (30), or midges. These findings support the hypothesis of the role of Anopheles in the transmission of R. felis; this hypothesis should be confirmed or refuted by future studies.
The clinical findings for R. felis infection are often unclear and are typically misdiagnosed as other febrile illnesses (12,31). Recently, the primary infection was described in a patient with polymorphous skin lesions, including papules, vesicles, erosions, and ulcers (12), similar to patients from Mexico (32). In the current study, a high incidence of R. felis infection was identified in children <15 years of age, as described (4). Fortunately, such patients improve rapidly with doxycycline treatment (12). For travelers to sub-Saharan Africa, the medications recommended for the chemoprophylaxis of malaria include doxycycline, which has the added advantage of being effective against rickettsioses (33).
This study showed the wide distribution and high incidence of R. felis infection; therefore, rickettsiosis should be considered one of the major causes of febrile diseases in sub-Saharan Africa. The demonstrated geographic distribution, seasonality, target population, incidence of relapses or re-infections, and asymptomatic infections of R. felis infection are similar to malaria. Further studies are needed to investigate the hypotheses that humans, as for epidemic typhus, another vector-borne relapsing rickettsiosis, or apes could be reservoirs and mosquitoes could be a vector for R. felis infection.
Dr Mediannikov is an infectious disease specialist and research scientist working at the Unit of Research on Emergent Infectious and Tropical Diseases in Marseille, France and Dakar, Senegal. His main research interests include vector-borne diseases and medical entomology.
Dr Socolovschi is a physician of infectious diseases and tropical medicine at the Medical School of Marseille, France. Her research interests focus on vector-borne infectious tropical diseases and medical entomology.
Acknowledgments
We thank the villagers who participated in this study. We also thank Masse Sambou, Aliou Diallo, Khadim Leye, Babacar Ndao, Malick Diop, Arsène Mabika, Marielle Bedotto, Denis Pyak and Annick Bernard for technical support.
This study was funded by the Agence National de Recherche grant 2010 (MALEMAF), Foundation Mediterranée Infection, Fondation Mérieux, and a collaborative grant to Josselin Thuilliez, University of Paris, Paris, France.
References
- Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–5. PubMed DOIGoogle Scholar
- Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–32. PubMed DOIGoogle Scholar
- Fenollar F, Mediannikov O, Socolovschi C, Bassene H, Diatta G, Richet H, Tropheryma whipplei bacteremia during fever in rural west Africa. Clin Infect Dis. 2010;51:515–21. PubMed DOIGoogle Scholar
- Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, Rickettsia felis-associated uneruptive fever, Senegal. Emerg Infect Dis. 2010;16:1140–2. PubMed DOIGoogle Scholar
- Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, Rickettsia felis infection in febrile patients, Western Kenya, 2007–2010. Emerg Infect Dis. 2012;18:328–31. PubMed DOIGoogle Scholar
- Richards AL, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16:1081–6. PubMed DOIGoogle Scholar
- Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Sokhna C, Bassene H, Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654. PubMed DOIGoogle Scholar
- Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol. 2011;49:3245–9. PubMed DOIGoogle Scholar
- Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–30. PubMed DOIGoogle Scholar
- Tourism Highlights United Nations World Tourism Organization [cited 2013 May 15]. http://mkt.unwto.org/sites/all/files/docpdf/unwtohighlights11enhr_3.pdf. 2011
- Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis. 2007;44:1560–8. PubMed DOIGoogle Scholar
- Mediannikov O, Fenollar F, Bassene H, Tall A, Sokhna C, Trape JF, Description of “yaaf”, the vesicular fever caused by acute Rickettsia felis infection in Senegal. J Infect. 2013;66:536-40. PubMed DOIGoogle Scholar
- Roucher C, Mediannikov O, Diatta G, Trape JF, Raoult D. A new Rickettsia species found in fleas collected from human dwellings and from domestic cats and dogs in Senegal. [PubMed ]. Vector Borne Zoonotic Dis. 2012;12:360–5. DOIPubMedGoogle Scholar
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. [PubMed]. Clin Microbiol Rev. 1997;10:694–719.PubMedGoogle Scholar
- Verhulst NO, Smallegange RC, Takken W. Mosquitoes as potential bridge vectors of malaria parasites from non-human primates to humans. Front Physiol. 2012;3:197. PubMed DOIGoogle Scholar
- Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci U S A. 2010;107:10561–6. PubMed DOIGoogle Scholar
- Socolovschi C, Pages F, Ndiath MO, Ratmanov P, Raoult D. Rickettsia species in African Anopheles mosquitoes. PLoS ONE. 2012;7:e48254. PubMed DOIGoogle Scholar
- Socolovschi C, Pages F, Raoult D. Rickettsia felis in Aedes albopictus mosquitoes, Libreville, Gabon. Emerg Infect Dis. 2012;18:1687–9. PubMed DOIGoogle Scholar
- Keita AK, Socolovschi C, Ahuka-Mundeke S, Ratmanov P, Butel C, Ayouba A, Molecular evidence for the presence of Rickettsia felis in the feces of wild-living African apes. PLoS ONE. 2013;8:e54679. PubMed DOIGoogle Scholar
- Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. [PubMed]. Am J Trop Med Hyg. 1994;51:123–37.PubMedGoogle Scholar
- Rolain JM, Bitam I, Buffet S, Marie JL, Bourry O, Portelli-Clerc C, Presence or absence of plasmid in Rickettsia felis depending on the source of fleas. Clin Microbiol Infect. 2009;Suppl 2:296–7. PubMed DOIGoogle Scholar
- Ndao M, Bandyayera E, Kokoskin E, Gyorkos TW, MacLean JD, Ward BJ. Comparison of blood smear, antigen detection, and nested-PCR methods for screening refugees from regions where malaria is endemic after a malaria outbreak in Quebec, Canada. J Clin Microbiol. 2004;42:2694–700. PubMed DOIGoogle Scholar
- Sigaúque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–13. PubMed DOIGoogle Scholar
- Okwara FN, Obimbo EM, Wafula EM, Murila FV. Bacteraemia, urinary tract infection and malaria in hospitalised febrile children in Nairobi: is there an association? East Afr Med J. 2004;81:47–51. PubMed DOIGoogle Scholar
- Phommasone K, Haris DH, Anantatat T, Castonguay-Vanier J, Keomany S, Souvannasing P, Concurrent infection with murine typhus and scrub typhus in southern Laos—the mixed and the unmixed. PLoS Negl Trop Dis. 2013;7:e2163. DOIPubMedGoogle Scholar
- Roucher C, Rogier C, Dieye-Ba F, Sokhna C, Tall A, Trape JF. Changing malaria epidemiology and diagnostic criteria for Plasmodium falciparum clinical malaria. PLoS ONE. 2012;7:e46188. PubMed DOIGoogle Scholar
- Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4. PubMed DOIGoogle Scholar
- Niang MN, Dosseh A, Ndiaye K, Sagna M, Gregory V, Goudiaby D, Sentinel surveillance for influenza in Senegal, 1996–2009. J Infect Dis. 2012;206(Suppl 1):S129–35. PubMed DOIGoogle Scholar
- Mediannikov O, Trape JF, Diatta G, Parola P, Fournier PE, Raoult D. Rickettsia africae, a neglected pathogen in West Africa. Emerg Infect Dis. 2010;16:571–3. PubMed DOIGoogle Scholar
- Mediannikov O, Audoly G, Diatta G, Trape JF, Raoult D. New Rickettsia sp. in tsetse flies from Senegal. [PubMed ]. Comp Immunol Microbiol Infect Dis. 2012;35:145–50. DOIPubMedGoogle Scholar
- Abdad MY, Stenos J, Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4:7168
- Zavala-Velázquez JE, Sosa-Ruiz JA, Zavala-Castro J, Jimenez-Delgadillo B, Vado-Solis IE, Sanchez-Elias RA, Rickettsia felis— the etiologic agent of three cases of rickettsiosis in Yucatan. Lancet. 2000;356:1079–80. PubMed DOIGoogle Scholar
- Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, Groves MG. Doxycycline propylaxis for human scrub typhus. J Infect Dis. 1982;146:811–8. PubMed DOIGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 11—November 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Didier Raoult, Université Aix-Marseille, URMITE, UMR CNRS 7278, IRD 198, INSERM 1095, Faculté de Médecine, 27 Bd Jean Moulin, 13385 Marseille Cedex 5 France
Top