Volume 20, Number 1—January 2014
CME ACTIVITY - Research
Raw Milk Consumption among Patients with Non–Outbreak-related Enteric Infections, Minnesota, USA, 2001–2010
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: December 19, 2013; Expiration date: December 19, 2014
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the problem of infections related to the use of raw milk products
• Distinguish the most common infection associated with raw milk consumption in the current study
• Evaluate clinical characteristics of individuals with infections related to raw milk consumption in the current study
• Compare sources of raw milk related to sporadic infection.
CME Editor
Claudia Chesley, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Claudia Chesley has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Trisha J. Robinson, MPH; Joni M. Scheftel, DVM, MPH; and Kirk E. Smith, DVM, PhD, have disclosed no relevant financial relationships.
Abstract
Raw milk has frequently been identified as the source of foodborne illness outbreaks; however, the number of illnesses ascertained as part of documented outbreaks likely represents a small proportion of the actual number of illnesses associated with this food product. Analysis of routine surveillance data involving illnesses caused by enteric pathogens that were reportable in Minnesota during 2001–2010 revealed that 3.7% of patients with sporadic, domestically acquired enteric infections had reported raw milk consumption during their exposure period. Children were disproportionately affected, and 76% of those <5 years of age were served raw milk from their own or a relative’s farm. Severe illness was noted, including hemolytic uremic syndrome among 21% of Escherichia coli O157–infected patients reporting raw milk consumption, and 1 death was reported. Raw milk consumers, potential consumers, and policy makers who might consider relaxing regulations regarding raw milk sales should be educated regarding illnesses associated with raw milk consumption.
Raw milk is well-established as a vehicle for numerous infectious diseases (1–8) and has frequently been identified as the source of outbreaks of foodborne illness (9,10). From 1998 through 2011, a total of 148 outbreaks were documented in the United States associated with the consumption of raw milk products, resulting in 2,384 illnesses, 284 hospitalizations, and 2 deaths (11). A recent report concluded that the incidence of reported outbreaks associated with raw dairy products was ≈150 times greater, per unit of product consumed, than the incidence involving pasteurized dairy products (12).
Although pasteurization has been available for use in the United States for over a century, a small proportion of the population continues to consume raw milk. In a 2006–2007 population survey comprising 10 states in the Foodborne Diseases Active Surveillance Network, 3.0% of respondents reported consuming raw milk during the previous 7 days (13). Farm families have traditionally been the primary consumers of raw milk, but evidence in recent years suggests that the population of raw milk consumers may be changing (14). Raw milk advocates tout raw milk for its purported health benefits and better taste, and many persons view raw milk consumption as an opportunity to support local dairies (15,16). Some raw milk advocates fail to acknowledge the elevated health risk associated with raw milk consumption and minimize the significance of reported outbreaks. In doing so, these advocates convey a false sense of the safety of raw milk to those who are considering consuming this product, and this sense of safety discourages a balanced assessment of the potential risks and benefits involved.
Although outbreaks associated with raw milk occur frequently and receive much media attention, the number of reported cases determined to be outbreak-related likely represents a small proportion of the actual number of illnesses associated with this product. Two lines of evidence support this assumption. First, among reported illnesses caused by enteric pathogens that are laboratory-confirmed, non–outbreak-related (i.e., sporadic) cases far outnumber those associated with recognized outbreaks (17). Second, for each reported laboratory-confirmed illness caused by a bacterial or protozoal enteric pathogen, an estimated 26–100 additional illnesses likely occur, depending on the pathogen (18). Therefore, any estimates of the number of illnesses associated with raw milk consumption should include an evaluation of sporadic cases, including multipliers to account for underdiagnosis and underreporting. However, little information is available on the number of sporadic cases of illness associated with raw milk consumption.
Minnesota is among the 30 US states that permit raw milk to be sold in some capacity (19), allowing for raw milk to be occasionally secured or purchased for personal use at the farm or place where the milk is produced (20). To better estimate the true number of human enteric pathogen infections associated with consumption of raw milk, we characterized sporadic enteric illnesses that occurred among patients in Minnesota who reported raw milk consumption during 2001–2010. Our primary objective was to provide better data on the true number of sporadically occurring disease cases associated with raw milk consumption.
Patients were identified through routine disease surveillance conducted at the Minnesota Department of Health (MDH). Infections caused by Campylobacter, Cryptosporidium, Shiga toxin–producing Escherichia coli (STEC, including O157 and non-O157 serogroups), and Salmonella are reportable to MDH by state rule, and active, population-based surveillance is conducted (21). All Minnesota residents with a laboratory-confirmed Campylobacter, Cryptosporidium, STEC O157, non-O157 STEC, or Salmonella infection are routinely interviewed by MDH staff using a disease-specific standard questionnaire about symptoms and food, water, animals, and other possible sources of infection during their exposure period. Each questionnaire contains a question about raw milk consumption, including where the raw milk was obtained and when it was consumed. Exposure period is defined as 7 days before illness onset for Campylobacter, STEC O157, non-O157 STEC, and Salmonella infections and 14 days for Cryptosporidium infection.
A case-patient was defined as a Minnesota resident who had a domestically acquired, laboratory-confirmed Campylobacter, Cryptosporidium, STEC O157, non-O157 STEC, or Salmonella infection and a specimen collection date during 2001–2010. Persons were excluded if they refused an interview or were unable to be reached for interview, were part of an outbreak, or traveled internationally during the exposure period. Patients infected with Campylobacter upsaliensis, Cryptosporidium hominis, and Salmonella enterica serotype Typhi were also excluded because these specific species or serotypes have not been documented to be associated with raw milk consumption or other cattle exposures. Patients infected with an unknown species or serotype were eligible for inclusion. The total number of case-patients reporting raw milk consumption was calculated, and we examined the demographic features, severity of illness, and raw milk sources among the case-patients. Estimates of the total number of illnesses that could be attributed to raw milk consumption were calculated by using published pathogen-specific multipliers that account for underdiagnosis (18).
Descriptive and univariate analyses were performed by using SAS 9.2 software (SAS Institute, Cary, NC, USA). Case-patients who refused to answer a question or responded don’t know or not sure were excluded from relevant analyses. Statistical significance was accepted at p<0.05.
During 2001–2010, a total of 20,034 Campylobacter, Cryptosporidium, STEC O157, non-O157 STEC, and Salmonella infections were reported to MDH. Among these cases, 6,695 were excluded for the following reasons: the patient reported international travel (2,648 cases) or refused or was unable to be reached for interview (1,530 cases); the patient was linked to a recognized outbreak (1,244 cases); or the infection was caused by a species or serotype not historically associated with raw milk or other cattle exposures (273 cases).
Of the excluded outbreak cases, 21 occurred during 5 recognized outbreaks associated with raw milk consumption in Minnesota during 2001–2010. These 5 outbreaks resulted in 7 hospitalizations and 1 case of hemolytic uremic syndrome (HUS). One outbreak of Campylobacter jejuni infections in 2001 was associated with raw milk consumption at a farm where a ministry group was staying. Two outbreaks of C. jejuni infections occurred in 2008: one was associated with raw milk consumption at a family reunion and the other with raw milk purchased from a local dairy farm. In 2010, an outbreak of STEC O157 infections and an outbreak of C. jejuni and Cryptosporidium parvum infections were associated with consumption of raw milk from the same dairy farm; both outbreaks included several cases associated with milk the consumers had picked up at illegal drop-off sites.
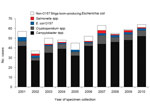
After exclusions, a total of 14,339 cases remained for analysis, including 6,747 Campylobacter spp., 1,742 Cryptosporidium spp., 1,069 STEC O157, 354 non-O157 STEC, and 4,427 Salmonella spp. cases. Among the 14,339 patients, 530 (3.7%) reported consumption of fluid raw milk during their exposure period (Table 1). The median annual number of case-patients reporting raw milk consumption was 53.5 (range 37–64), but this number generally increased over time (Figure 1). Among the 273 persons who were excluded from study because of infection with a species or serotype not historically associated with raw milk or other cattle exposures, only 2 (0.7%) reported raw milk consumption; this was significantly lower than the 3.7% of cases included in the analysis (p = 0.01).
Persons with Campylobacter infection had the highest percentage of reported raw milk consumption (6.0%), and Campylobacter spp. accounted for 407 (77%) of the 530 cases with reported raw milk consumption (Table 1, Figure 1). Among case-patients infected with other pathogens, the percentage, by pathogen, reporting raw milk consumption included: non-O157 STEC, 3.4%; Cryptosporidium spp., 3.0%; STEC O157; 1.8%, and Salmonella spp., 0.9%. The following data were available regarding the speciation of pathogens from case-patients: 378 Campylobacter isolates (C. jejuni, 96.8%; C. coli, 2.6%; and C. lari, 0.5%); 23 Cryptosporidium parvum specimens; 36 Salmonella isolates (16 serotypes, most frequently S. enterica serotype Typhimurium, 10 isolates; S. enterica serotype Montevideo, 6 isolates; and S. enterica serotype Newport, 5 isolates).
Twelve patients were co-infected with Campylobacter spp. and 1 other enteric pathogen: 9 with Cryptosporidium spp., 1 with STEC O157, 1 with non-O157 STEC, and 1 with Salmonella spp. In addition, 1 patient who was reported to have consumed raw milk was infected with different pathogens at different times during the study period: STEC O157 (including HUS) at 1 year of age and Salmonella spp. 1 year later.
Male case-patients comprised 62.6% of study participants reporting raw milk consumption (Table 1). Case-patients reporting raw milk consumption were more likely than the average Minnesotan to be white (96.5% vs. 85.3%; p<0.001), and 96.8% were non-Hispanic. Overall, the median age of case-patients reporting raw milk consumption was 17 years (range 9 months to 92 years); 25% were <5 years of age, 38% were <10 years of age, and 59% were <20 years of age (Figure 2). Among patients with STEC O157 infections, those reporting raw milk consumption had a median age of 5 years (range 11 months to 63 years), compared with a median age of 16 years (range 5 months to 92 years) among those who did not report raw milk consumption (p = 0.02). Likewise, patients with non-O157 STEC infections who reported raw milk consumption had a median age of 4 (range 1–63) years. Patients with Cryptosporidium, Salmonella, or Campylobacter infection who reported raw milk consumption had median ages of 9, 16, and 18 years, respectively. Illnesses occurring among patients reporting raw milk consumption were disproportionately distributed throughout the year; 35% (186/530) of specimen collection dates occurred during the months of June, July, or August (p<0.001).
Seventy (13%) case-patients reporting raw milk consumption required hospitalization for a median of 3 (range 2–27) days for their illness. HUS occurred among 4 (21%) of the 19 patients with STEC O157 infection, including 2 of 4 children <3 years of age. One (8%) of the 12 patients with non-O157 STEC infection developed HUS. An 11-month-old infant with STEC O157 infection died.
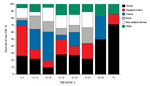
Of the 530 case-patients who consumed raw milk, 377 (71%) provided information on the source of their milk, and almost half either obtained it from their own dairy farm (91 consumers, 24%) or from a relative’s dairy farm (90 consumers, 24%). Other reported sources included friends (73 consumers, 19%); nonrelative farmers, including direct farm sales (50 consumers, 13%); the workplace or a relative’s workplace (39 consumers, 10%); neighbors (16 consumers, 4%); drop-off sites (7 consumers, 2%); or other sources, including at daycare or school (11 consumers, 3%). Those who reported consuming raw milk from their own dairy farm or from a relative’s dairy farm were significantly younger than those who reported obtaining raw milk from a nonfamily source (median age 9 years vs. 19 years; p<0.001). Among children <5 years of age, 76% were reported to have consumed raw milk from their own dairy farm or a relative’s dairy farm; this proportion declined with age among pediatric patients, and a steep drop to 9% occurred among those 17–20 years of age, who more frequently reported obtaining raw milk from friends or at work (Figure 3). Among 464 case-patients with known information, 232 (50%) also reported contact with cattle or their environment during the exposure period; 68% of these exposures occurred in persons living or working on a farm or visiting a family member’s farm.
Pathogen-specific underdiagnosis multipliers (18) were applied to data regarding the 530 cases associated with reported domestic raw milk consumption and resulted in an estimate that 20,502 Minnesotans became ill with sporadic Campylobacter, Cryptosporidium, STEC O157, non-O157 STEC, or Salmonella infection during 2001–2010 after drinking raw milk (Table 2). We applied the percentage of Minnesotans in the Foodborne Diseases Active Surveillance Network population survey who reported consuming raw milk (2.3%) (13) to the 2006 state population (5,167,101) and determined that an estimated 118,843 Minnesotans consume raw milk during a given week. If these projected illness data are applied to the projected number of raw milk consumers and the percentage of Minnesotans who consume raw milk was consistently 2.3% during the study period, then an estimated 17.3% of raw milk consumers in Minnesota may have acquired an illness caused by 1 of these enteric pathogens during the 10-year study period.
Our study quantifies the number of non–outbreak-related enteric illnesses that could be associated with raw milk consumption. The results indicate that the number of sporadic raw milk–associated illnesses is likely substantial, greatly exceeding the number of cases linked to recognized raw milk–associated outbreaks. Furthermore, the number of cases associated with reported raw milk consumption appears to be increasing, just as the movement to relax regulation of raw milk sales appears to be gaining momentum in many states.
We found that young children were disproportionately affected, and the source of the raw milk they consumed was often their own dairy farm or a relative’s farm. This reinforces the risks associated with raw milk consumption for all young children, including those who live on or visit farms. Half of the STEC O157–related and non-O157 STEC–related illnesses occurred among children <5 years of age; this finding is of particular concern because of the increased risk for HUS among this age group (22). In our study, HUS developed in 21% of patients with STEC O157 infections who reported drinking raw milk, and 1 of these patients died. Because of the potential for severe health consequences associated with the consumption of raw milk, the American Academy of Pediatrics and several other professional organizations have adopted a position statement advising that children should only consume milk products that are pasteurized (23).
Our study suggests that farm family members, particularly young children, who consume raw milk are susceptible to illness from it. Results of previous studies have shown that 30%–50% of dairy producers surveyed were unaware that their raw bulk tank milk could contain disease-causing microorganisms (24,25). Therefore, dairy farm families, in particular, should be educated regarding this issue. The differences in disease risk by age group may be a result of acquired immunity among older adolescents and adults who were exposed to pathogens during farm exposures in childhood (26).
Most raw milk consumers included in our study were infected with Campylobacter spp., and these bacteria have repeatedly been associated with raw milk consumption, both in outbreak investigations and in case–control studies (27,28). Because of the complexities involved with molecular subtyping for Campylobacter spp. (29) and because of the high proportion of patients who do not report a source for their raw milk, it is likely that some of the apparently sporadic infections associated with raw milk consumption are actually illnesses related to unrecognized outbreaks.
This study provides evidence that raw milk is also a vehicle for Cryptosporidium spp., the second most common pathogen identified among raw milk drinkers. Cryptosporidiosis is a common infection in cattle, and contact with infected animals or their environment is frequently associated with human cryptosporidiosis (30,31). Although few cryptosporidiosis outbreaks associated with raw milk consumption have been reported (6), findings of other studies include an association between raw milk consumption and cryptosporidiosis (32,33).
We noted an increase in enteric disease cases associated with reported raw milk consumption during the summer months (June–August) compared with other seasons. This trend is in sync with the general seasonality of the enteric pathogens included in this study, and it is also consistent with data from recent studies on the seasonal incidence of Salmonella spp. sampled from bulk tank milk, dairy cows, and farm environments (34) and the seasonal trend of fecal shedding of STEC O157 by dairy cattle (35).
This study had several potential limitations. First, the cases were sporadic, so illness among the raw milk consumers could not be definitively linked to their raw milk consumption without overestimating the number of illnesses associated with raw milk consumption. For example, illness among some patients could have been associated with direct contact with cattle rather than raw milk consumption. However, 50% of patients did not report cattle contact, and the high-risk nature of raw milk consumption makes it the most likely source for most of the patients who did report concomitant cattle contact. This conclusion is supported by the close demographic and illness similarities among patients with reported raw milk consumption in our study and the outbreak cases reported by Langer et al. (12). It is also supported by the very low (0.7%) background level of raw milk consumption among patients with pathogens of a species or serotype not historically associated with raw milk consumption or other cattle exposures.
The second potential limitation is that not all specimens of the pathogens of interest were identified to species or serotype; this was most pertinent for Cryptosporidium spp. Therefore, it is possible that some patients were misclassified as eligible, thus underestimating the percentage of raw milk drinkers. The third potential limitation is that refusals to participate in an interview or to answer questions about raw milk could be biased toward raw milk consumers. In contrast to the first limitation, this could potentially have resulted in an underestimation of the number of illnesses associated with raw milk consumption. The fourth potential limitation is that the data available to us for this study did not permit estimation of a true absolute risk for illness associated with raw milk consumption. The fifth and last potential limitation is that the pathogen-specific underdiagnosis multipliers have confidence intervals that were not considered here; therefore, estimates of raw milk–associated illnesses could be substantially higher or lower than we have reported (18).
Sporadic cases of illness associated with raw milk consumption far outnumber cases associated with recognized outbreaks. During the study period, the number of patients with sporadic laboratory-confirmed infections who reported raw milk consumption (n = 530) was 25 times greater than the number of raw milk–associated outbreak cases (n = 21) among Minnesota residents. Furthermore, we estimated that up to 20,502 Minnesotans, or 17% of raw milk consumers, may have become ill with enteric pathogens during the study period after consuming raw milk. This finding suggests that outbreaks represent a small number of the illnesses associated with raw milk consumption and that the risk for illness associated with raw milk consumption is far greater than determined based on the occurrence of recognized outbreaks. Findings such as ours should be used to further educate potential raw milk consumers, as well as policy makers who might be asked by constituents to relax regulations regarding raw milk sales.
Ms Robinson is an epidemiologist with the Minnesota Department of Health. Her research interests include the surveillance and prevention of enteric illnesses.
Acknowledgments
We thank the many staff members of the Foodborne, Vectorborne, and Zoonotic Diseases Unit at the Minnesota Department of Health who helped collect data used in this report.
This work was supported in part through cooperative agreements (5 U01 EH000698-03 and U50/CCU511190) with the Centers for Disease Control and Prevention as part of the Environmental Health Specialist’s Network and the Emerging Infections Program, Foodborne Diseases Active Surveillance Network.
References
- Centers for Disease Control and Prevention. Human tuberculosis caused by Mycobacterium bovis—New York City, 2001–2004. MMWR Morb Mortal Wkly Rep. 2005;54:605–8.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Outbreak of Campylobacter jejuni infections associated with drinking unpasteurized milk procured through a cow-leasing program—Wisconsin, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:548–9.PubMedGoogle Scholar
- Guh A, Phan Q, Nelson R, Purviance K, Milardo E, Kinney S, Outbreak of Escherichia coli O157 associated with raw milk, Connecticut, 2008. Clin Infect Dis. 2010;51:1411–7. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multistate outbreak of Salmonella serotype Typhimurium infections associated with drinking unpasteurized milk—Illinois, Indiana, Ohio, and Tennessee, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:613–5.PubMedGoogle Scholar
- Denny J, Bhat M, Eckman K. Outbreak of Escherichia coli O157:H7 associated with raw milk consumption in the Pacific Northwest. Foodborne Pathog Dis. 2008;5:321–8. DOIPubMedGoogle Scholar
- Harper CM, Cowell NA, Adams BC, Langley AJ, Wohlsen TD. Outbreak of Cryptosporidium linked to drinking unpasteurised milk. Commun Dis Intell Q Rep. 2002;26:449–50.PubMedGoogle Scholar
- Osterholm MT, MacDonald KL, White KE, Wells JG, Spika JS, Potter ME, An outbreak of a newly recognized chronic diarrhea syndrome associated with raw milk consumption. JAMA. 1986;256:484–90. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multi-state outbreak of yersiniosis. MMWR Morb Mortal Wkly Rep. 1982;31:505–6.PubMedGoogle Scholar
- Headrick ML, Korangy S, Bean NH, Angulo FJ, Altekruse SF, Potter ME, The epidemiology of raw milk–associated foodborne disease outbreaks reported in the United States, 1973 through 1992. Am J Public Health. 1998;88:1219–21. DOIPubMedGoogle Scholar
- Lejeune JT, Rajala-Schultz PJ. Unpasteurized milk: a continued public health threat. Clin Infect Dis. 2009;48:93–100. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Food safety. Raw milk questions and answers. 2013 [cited 2013 Apr 22]. http://www.cdc.gov/foodsafety/rawmilk/raw-milk-questions-and-answers.html
- Langer AJ, Ayers T, Grass J, Lynch M, Angulo FJ, Mahon BE. Nonpasteurized dairy products, disease outbreaks, and state laws—United States, 1993–2006. Emerg Infect Dis. 2012;18:385–91. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Foodborne Diseases Active Surveillance Network (FoodNet) population survey atlas of exposures. 2006–2007 [cited 2012 May 1]. http://www.cdc.gov/foodnet/surveys/FoodNetExposureAtlas0607_508.pdf
- Katafiasz AR, Bartlett P. Motivation for unpasteurized milk consumption in Michigan, 2011. Food Protection Trends. 2012;32:124–8.
- Leedom JM. Milk of nonhuman origin and infectious diseases in humans. Clin Infect Dis. 2006;43:610–5. DOIPubMedGoogle Scholar
- Jay-Russell MT. Raw (unpasteurized) milk: are health-conscious consumers making an unhealthy choice? Clin Infect Dis. 2010;51:1418–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food–foodborne diseases active surveillance network, 10 US sites, 1996–2010. MMWR Morb Mortal Wkly Rep. 2011;60:749–55.PubMedGoogle Scholar
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15.PubMedGoogle Scholar
- National Association of State Departments of Agriculture. NASDA releases raw milk survey. 2011 [cited 2012 March 2]. http://www.nasda.org/file.aspx?id=3916
- Minnesota Statutes 2013, section 32.393, subdivision 1. Pasteurization. 2013 [cited 2013 May 1] https://www.revisor.mn.gov/statutes/?id=32.393
- Minnesota Department of Health. Communicable disease rule, chapter 4605. 2012 May 29 [cited 2013 May 1]. http://www.health.state.mn.us/divs/idepc/dtopics/reportable/rule/rule.html
- Tserenpuntsag B, Chang HG, Smith PF, Morse DL. Hemolytic uremic syndrome risk and Escherichia coli O157:H7. Emerg Infect Dis. 2005;11:1955–7. DOIPubMedGoogle Scholar
- Bradley J, Pickering LK, Jareb J. Advise families against giving children unpasteurized milk. AAP News. 2008 Dec 2. 2008;29:29.
- Jayarao BM, Donaldson SC, Straley BA, Sawant AA, Hegde NV, Brown JL. A survey of foodborne pathogens in bulk tank milk and raw milk consumption among farm families in Pennsylvania. J Dairy Sci. 2006;89:2451–8. DOIPubMedGoogle Scholar
- Hoe FG, Ruegg PL. Opinions and practices of Wisconsin dairy producers about biosecurity and animal well-being. J Dairy Sci. 2006;89:2297–308. DOIPubMedGoogle Scholar
- Kassenborg HD, Hedberg CW, Hoekstra M, Evans MC, Chin AE, Marcus R, Farm visits and undercooked hamburgers as major risk factors for sporadic Escherichia coli O157:H7 infection: data from a case-control study in 5 FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S271–8. DOIPubMedGoogle Scholar
- Wood RC, MacDonald KL, Osterholm MT. Campylobacter enteritis outbreaks associated with drinking raw milk during youth activities. A 10-year review of outbreaks in the United States. JAMA. 1992;268:3228–30. DOIPubMedGoogle Scholar
- Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–96. DOIPubMedGoogle Scholar
- Hedberg CW, Smith KE, Besser JM, Boxrud DJ, Hennessy TW, Bender JB, Limitations of pulsed-field gel electrophoresis for the routine surveillance of Campylobacter infections. J Infect Dis. 2001;184:242–4. DOIPubMedGoogle Scholar
- Santín M, Trout JM, Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet Parasitol. 2008;155:15–23. DOIPubMedGoogle Scholar
- Yoder JS, Beach MJ. Cryptosporidium surveillance and risk factors in the United States. Exp Parasitol. 2010;124:31–9. DOIPubMedGoogle Scholar
- Thomson MA, Benson JW, Wright PA. Two year study of Cryptosporidium infection. Arch Dis Child. 1987;62:559–63. DOIPubMedGoogle Scholar
- Fretz R, Svoboda P, Ryan UM, Thompson RC, Tanners M, Baumgartner A. Genotyping of Cryptosporidium spp. isolated from human stool samples in Switzerland. Epidemiol Infect. 2003;131:663–7. DOIPubMedGoogle Scholar
- Pangloli P, Dje Y, Ahmed O, Doane CA, Oliver SP, Draughon FA. Seasonal incidence and molecular characterization of Salmonella from dairy cows, calves, and farm environment. Foodborne Pathog Dis. 2008;5:87–96. DOIPubMedGoogle Scholar
- Hussein HS, Bollinger LM. Prevalence of Shiga toxin–producing Escherichia coli in beef cattle. J Food Prot. 2005;68:2224–41.PubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Raw Milk Consumption among Patients with Non–Outbreak-related Enteric Infections, Minnesota, USA, 2001–2010
CME Questions
1. You are seeing a pair of brothers, ages 4 and 10 years, for a 2-day history of fever and loose stools. The boys’ mother explains that they had been drinking raw milk for the past week. What should you consider regarding foodborne illness associated with the consumption of raw milk?
A. Outbreaks of illness account for many more cases of foodborne illness associated with raw milk compared with sporadic cases
B. The risk for illness with raw dairy products is approximately 150 times that associated with pasteurized products
C. Public health records accurately describe the prevalence of sporadic foodborne illness
D. Less than 1% of cases in the current study reported raw milk exposure before their infection
2. What was the most common infection associated with raw milk consumption in the current study?
A. Cryptosporidium species
B. Campylobacter species
C. Salmonella species
D. Escherichia coli
3. As you evaluate these patients, what should you consider regarding the clinical presentation of patients with illness related to raw milk in the current study?
A. Infections affected both genders equally
B. One-quarter of affected individuals were younger than 5 years
C. Hispanic adults were particularly at risk for infection
D. Cases were spread equally throughout the year
4. What was the principal source of raw milk among individuals infected in the current study?
A. Urban farmers’ markets
B. Unlicensed roadside farm stands
C. Their own dairy farm or a relative’s dairy farm
D. Daycare or school
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 20, Number 1—January 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence. Trisha J. Robinson, Minnesota Department of Health, PO Box 64975, St. Paul, MN 55164, USAAddress for correspondence. Trisha J. Robinson, Minnesota Department of Health, PO Box 64975, St. Paul, MN 55164, USA
Top