Volume 20, Number 2—February 2014
Dispatch
Melioidosis Caused by Burkholderia pseudomallei in Drinking Water, Thailand, 2012
Abstract
We identified 10 patients in Thailand with culture-confirmed melioidosis who had Burkholderia pseudomallei isolated from their drinking water. The multilocus sequence type of B. pseudomallei from clinical specimens and water samples were identical for 2 patients. This finding suggests that drinking water is a preventable source of B. pseudomallei infection.
Burkholderia pseudomallei is a Tier 1 select agent and the cause of naturally acquired melioidosis in Southeast Asia, northern Australia, the Indian subcontinent, and areas of South America (1). The organism is present in soil and surface water, and most melioidosis cases are believed to result from bacterial inoculation or inhalation (1). Ingestion has been increasingly suspected to be an alternative route of infection. B. pseudomallei isolated from a community water supply in Australia was genetically identical to that causing disease in clusters (2,3), although no direct evidence was available to show that affected cases had consumed contaminated water. The pattern of infection after ingestion in an experimental model includes multiple organ dissemination and hepatosplenic abscesses, which are common features of human melioidosis and supportive evidence for ingestion as a route of human infection (4).
Our previous study in Ubon Ratchathani Province in northeastern Thailand investigated the activities of daily living associated with acquisition of melioidosis (5). Households of participants who resided ≤100 km of Sappasithiprasong Hospital in Ubon Ratchathani were visited, and water samples were collected from all sources of drinking water and from tap water (5). Culture of these samples for B. pseudomallei provided borderline statistical evidence to suggest that consuming water containing B. pseudomallei was associated with melioidosis (conditional odds ratio 2.2, 95% CI 0.8–5.8, p = 0.08) (5). We performed a study to further evaluate the role of ingestion as a route of infection, and we identified the genotypes of B. pseudomallei isolated from patients and the water supplies consumed by them.
During July 2010–December 2011, we collected and cultured 576 water samples from the households of 142 case-patients and 288 controls (5). In brief, 5 L of water was collected from each source of drinking water and tap water, regardless of consumption. If the water was filtered or boiled by the householder before consumption, samples were collected for culture. Locations from which water samples were collected were recorded by using the Epicollect Program (6). For each sample, 1 L was passed through two 0.45-μm filters (500 mL through each filter), and the remaining 4 L was passed through 2.5 g of sterile diatomaceous earth (Celite; World Minerals Corporation, San Jose, CA, USA).
Filters were cultured on Ashdown agar to obtain a quantitative bacterial count, and diatomaceous earth was cultured in selective broth containing (15 mL of threonine–basal salt plus colistin broth) to obtain a sensitive, qualitative method. Broth was incubated at 40°C in air for 48 h, after which 10 μL of the upper layer was streaked onto an Ashdown agar plate to achieve single colonies, incubated at 40°C in air, and examined every 24 h for 7 days. If enrichment broth cultures showed positive results but filters on Ashdown agar showed negative results, the quantitative count was defined as <1 CFU/L. Ten colonies of B. pseudomallei were randomly picked from the primary plate and saved for genotyping. If there were <10 colonies, all primary plate colonies were used, and the number was adjusted to 10 after subculture of the enrichment broth.
Genotyping was performed by using pulsed-field gel electrophoresis and multilocus sequence typing (MLST) as described (7,8). In brief, isolates from clinical and water samples from the same patient were subjected to electrophoresis on the same gel. Colonies with an identical banding pattern were classified as the same genotype, and colonies with ≥1 different patterns were further genotyped by using MLST (8). A map was drawn by using the R program (www.r-project.org/) and OpenStreetMap (www.openstreetmap.org) data.
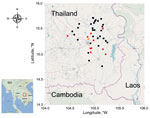
A total of 43 (7%) of 576 water samples were culture positive for B. pseudomallei (Table 1). The rate of positivity did not differ between the rainy season (June–November) and dry season (December–May) (8% vs. 6%; p = 0.32, by Fisher exact test) (Figure 1). Positive water samples were geographically distributed across the sampling area (Figure 2). The median quantitative count of B. pseudomallei in water was 1 CFU/L (interquartile range <1–13 CFU/L, range <1–65 CFU/L) (Table 1). Of the 43 culture-positive water samples, 21 (7%) of 288 were from control households and 22 (15%) of 142 were from case-patient households. Ten of these case-patients with melioidosis reported drinking from contaminated water sources in the 30 days before the onset of illness.
We compared genotypes of B. pseudomallei in clinical specimens and water samples from the 10 case-patients who consumed water that was subsequently shown to contain B. pseudomallei. A total of 91 colonies from 10 water samples and 1 colony isolated from blood culture (7) or sputum (3) from each case-patient was examined by using pulsed-field gel electrophoresis and MLST. The median number of different genotypes observed per water sample was 3 (range 1–6). Two case-patients were infected with a B. pseudomallei genotype that was also present in their drinking water (Table 2).
Our finding that drinking water, including public tap water, in northeastern Thailand contains viable B. pseudomallei is a public health concern. Public tap water in Thailand, to which melioidosis is highly endemic, needs to be safe and free from B. pseudomallei contamination. B. pseudomallei can survive in water for prolonged periods (9). The National Tap Water Quality Assurance Program in Thailand currently does not include B. pseudomallei detection (10) and this fact warrants review. Unlike observations in Hong Kong (11), all collected rainwater specimens were culture negative for B. pseudomallei. The reasons for this finding are not known, but it may be that the bacterial count was below the detection limit.
Clusters of melioidosis cases with the same genotype in Australia might be related to a temporary stoppage of the water purification process (2,3). Clustering has not been reported from Thailand, and might be explained by the high degree of genetic diversity of B. pseudomallei in water in this setting (12). The validity of a study that reported the detection of B. pseudomallei in 6 (7%) of 85 drinking water samples in Italy is questionable (13) because the bacterial isolates were not confirmed to be B. pseudomallei by using specific identification methods, and there have been no reported cases of indigenous melioidosis in Italy (14).
We propose that ingestion was the probable route of melioidosis acquisition in the 2 patients who each had matching B. pseudomallei genotypes in their clinical sample and drinking water. Although other routes of infection, such as inoculation injury, are possible for these 2 patients, genetic diversity of B. pseudomallei in even a small area in Thailand indicates that the likelihood of observing an identical genotype for an isolate in water and an isolate from a human that was acquired by soil inoculation is probably low (12). Infection with >1 strain of B. pseudomallei is rare (15), but we might not have linked clinical and water strains in some cases because we did not pick appropriate colonies for genotyping from a water sample that contained multiple genotypes. In summary, evidence from a case–control study (5) and our molecular study suggests that most B. pseudomallei infections are acquired by inoculation, but a proportion of cases are caused by ingestion, and such cases are potentially preventable.
Dr Limmathurotsakul is a clinician scientist at the Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Bangkok, Thailand. His research interests are the epidemiology of melioidosis and Burkholderia pseudomallei in the environment.
Acknowledgments
We thank the patients and staff at Sappasithiprasong Hospital for assistance; the Wellcome Trust–Oxford University–Mahidol University Tropical Medicine Research Program for assistance; and Vanaporn Wuthiekanun, Premjit Amornchai, Areeya Faosap, Maliwan Hongsuwan, Suparinya Hanvongsa, Duangporn Narin, Mayura Malasit, Nittayasee Wongsuwan, Varinthorn Praikaew, and Jittana Suwannapruk for technical assistance.
This study was supported by a project grant (090219/Z/09/Z) from the Wellcome Trust. S.J.P. is supported by the National Institute for Health Research Cambridge Biomedical Research Centre.
References
- Limmathurotsakul D, Peacock SJ. Melioidosis: a clinical overview. Br Med Bull. 2011;99:125–39 . DOIPubMedGoogle Scholar
- Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O’Reilly L, Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–9 .PubMedGoogle Scholar
- Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg. 2001;65:177–9 .PubMedGoogle Scholar
- West TE, Myers ND, Limmathurotsakul D, Liggitt HD, Chantratita N, Peacock SJ, Pathogenicity of high-dose enteral inoculation of Burkholderia pseudomallei to mice. Am J Trop Med Hyg. 2010;83:1066–9. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Kanoksil M, Wuthiekanun V, Kitphati R, deStavola B, Day N, Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case control study. PLoS Negl Trop Dis. 2013;7:e2072. DOIPubMedGoogle Scholar
- Aanensen DM, Huntley DM, Feil EJ, al-Own F, Spratt BG. EpiCollect: linking smartphones to web applications for epidemiology, ecology and community data collection. PLoS ONE. 2009;4:e6968. DOIPubMedGoogle Scholar
- Maharjan B, Chantratita N, Vesaratchavest M, Cheng A, Wuthiekanun V, Chierakul W, Recurrent melioidosis in patients in northeast Thailand is frequently due to reinfection rather than relapse. J Clin Microbiol. 2005;43:6032–4. DOIPubMedGoogle Scholar
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–79. DOIPubMedGoogle Scholar
- Pumpuang A, Chantratita N, Wikraiphat C, Saiprom N, Day NP, Peacock SJ, Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans R Soc Trop Med Hyg. 2011;105:598–600. DOIPubMedGoogle Scholar
- Provincial Waterworks Authority of Ubon Ratchathani. Thailand. The national tap water quality assurance program [cited 2012 Feb 11]. http://www.pwa.co.th/province/cgi-bin/index.php?Province=34.
- Kinoshita R. Epidemiology of melioidosis in an oceanarium: a clinical, environmental and molecular study. Hong Kong: University of Hong Kong; 2003.
- Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NP, Peacock SJ. Burkholderia pseudomallei is genetically diverse in agricultural land in northeast Thailand. PLoS Negl Trop Dis. 2009;3:e496. DOIPubMedGoogle Scholar
- Zanetti F, De Luca G, Stampi S. Recovery of Burkholderia pseudomallei and B. cepacia from drinking water. Int J Food Microbiol. 2000;59:67–72. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis. 2009;3:e496 .PubMedGoogle Scholar
- Wuthiekanun V, Limmathurotsakul D, Chantratita N, Wongsuvan G, Thanwisai A, Biaklang M, Simultaneous infection with more than one strain of Burkholderia pseudomallei is uncommon in human melioidosis. J Clin Microbiol. 2007;45:3830–2 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 20, Number 2—February 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Direk Limmathurotsakul, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Rd, Bangkok 10400, ThailandDirek Limmathurotsakul, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Rd, Bangkok 10400, Thailand
Top