Volume 20, Number 7—July 2014
Synopsis
Epidemiology, Clinical Manifestations, and Outcomes of Streptococcus suis Infection in Humans
Abstract
Streptococcus suis, a bacterium that affects pigs, is a neglected pathogen that causes systemic disease in humans. We conducted a systematic review and meta-analysis to summarize global estimates of the epidemiology, clinical characteristics, and outcomes of this zoonosis. We searched main literature databases for all studies through December 2012 using the search term “streptococcus suis.” The prevalence of S. suis infection is highest in Asia; the primary risk factors are occupational exposure and eating of contaminated food. The pooled proportions of case-patients with pig-related occupations and history of eating high-risk food were 38.1% and 37.3%, respectively. The main clinical syndrome was meningitis (pooled rate 68.0%), followed by sepsis, arthritis, endocarditis, and endophthalmitis. The pooled case-fatality rate was 12.8%. Sequelae included hearing loss (39.1%) and vestibular dysfunction (22.7%). Our analysis identified gaps in the literature, particularly in assessing risk factors and sequelae of this infection.
Streptococcus suis is a neglected zoonotic pathogen that has caused large outbreaks of sepsis in China (1,2) and has been identified as the most common and the third leading cause of bacterial meningitis in adults in Vietnam and Hong Kong, respectively (3–5). S. suis infection is acquired from pigs, either during slaughtering or by handling and eating undercooked pork products. It is potentially preventable (3,6). Epidemiology of the infection differs between Western and Asian regions (7), and the role of high-risk eating habits (i.e., ingesting raw or undercooked pig parts, including pig blood, organs, and meat) in some Asian communities recently has been recognized (6,8,9). Rates of S. suis infection are low in the general populations of Europe and North America, and cases are concentrated among occupationally exposed groups, including abattoir workers, butchers, and pig breeders (10,11).
In a 2009 review, ≈700 S. suis infections were reported worldwide by 2009, mostly from China and Vietnam (12). Clinical characteristics of this infection have been reviewed (12,13) and include meningitis, sepsis, endocarditis, arthritis, hearing loss, and skin lesions. Treatment of S. suis infection requires ≈2 weeks of intravenous antimicrobial drugs (12). The death rate varies, and deafness is a common sequela in survivors.
Although substantial new data on the incidence, clinical and microbiological characteristics, and risk factors for S. suis infection have accumulated during recent years, the prevalence of this infection has not measurably decreased. We conducted a systematic review and meta-analysis to update the evidence and summarize the estimates of epidemiologic and clinical parameters to support practitioners’ and policy makers’ efforts to prevent and control this infection.
We conducted the review in accordance with recommendations of the PRISMA statement (14). The protocol for this review has been registered at PROSPERO International prospective register of systematic reviews (no. CRD42011001742).
Search Strategy and Selection Criteria
We systematically and inclusively searched 5 main electronic databases (PubMed, Scopus, ISI Web of Science, Science Direct, and Google Scholar) for all studies published until the end of December 2012 (Figure 1). The following search term was used as a text word in each database, as follows: PubMed—“streptococcus suis” in all fields, limited to humans; Scopus—“streptococcus suis” in all fields, excluding veterinary medicine articles; ISI Web of Science—“streptococcus suis” in topic with exclusion of veterinary science areas; Science Direct—“streptococcus suis” in all fields, with articles in veterinary medicine journals excluded; and Google Scholar—“allintitle: ‘streptococcus suis’”
We also searched using the same search term “streptococcus suis” in other databases, including Virtual Health Library, SIGLE, WHOLIS, LILACS, IMSEAR-HELLIS, and China Academic Journals Full-text Database and checked the reference lists of retrieved articles. We did not restrict the types of studies and publication languages, and non-English papers were translated for review. Publications were excluded if they did not report any human cases of S. suis infection, reported data that overlapped with already included articles and provided no additional information, reported cases without information indicating the location of the patients, or reported data that could not be reliably extracted.
Data Extraction
Two reviewers (N.H. and V.T.L.H.) independently screened the titles and abstracts, and examined the full-text publications by using identical selection criteria and data abstraction forms. The results of data extraction showed a high degree of agreement between the reviewers (κ>0.90 for the main variables). Any disagreements were resolved by discussion and consensus between the reviewers and other authors (N.T. Huy, H.W., P.H., K.H.). We emailed the original authors of the articles that contained ambiguous data (1 email attempt per author) for clarification, and the ambiguous data were excluded from analyses if we did not receive a response.
Data extracted included year of publication, year of data collection, study design, data collection approach, country of origin, hospital where the patients were recruited, patient characteristics, clinical manifestations, methods of diagnosis, clinical and laboratory parameters, outcomes, and histories.
Analyses
We described the relevant epidemiologic and clinical factors using count for number of cases, proportions with 95% CIs for categorical factors (sex, occupation, exposure, history), and mean with SD for continuous factors (age, duration, and laboratory parameters). Event rates are presented as proportions with 95% CIs for signs, symptoms, and outcomes. We defined an event rate as the ratio of number of events to the number of all patients assessed in each study.
We pooled all single cases from the publications that reported <5 cases into 1 dataset and produced summary outputs, which were then meta-analyzed with other large studies (reporting >5 cases). We report the values of reviewed factors in 3 sets: summary values from the single-case dataset, median values (range) of the large studies, and pooled values from the meta-analysis as appropriate.
Meta-analysis was conducted by using Comprehensive Meta-analysis software version 2 (Biostat, Englewood, NJ, USA; http://www.Meta-Analysis.com) when >2 studies reported the reviewed factor. We tested heterogeneity using the Q statistic and I2 test (15). Pooled values and 95% CIs were generated from a fixed-effects model or from a random-effects model, and each was study weighted by the inverse of that study’s variance. We used the fix-effects model when heterogeneity was not significant and a random-effects model when heterogeneity was evident (16). Median (range) was converted to mean (SD) by using proposed formulas (17), and interquartile ranges to SDs and subgroup values to total values by Cochrane suggested methods (18).
We assessed publication bias using funnel plots and the Egger’s regression test (if >10 studies were included in the meta-analysis). If publication bias was found, the Duvall and Tweedie trim and fill method was performed to add possible missing studies to improve the symmetry and calculate the adjusted pooled values (19). We used subgroup analyses (when >10 studies were included) and bivariate meta-regression (when >20 studies were included) to examine the effect of study-level variables, including year of publication (2005 and earlier vs. after 2005 [because the Sichuan outbreak occurred in 2005]), study design (case series, outbreak investigation, cross-sectional), location (China mainland, Hong Kong, Thailand, Vietnam, and others), data collection (retrospective vs. prospective) and meningitis rate (<50%, 50%–90%, and >90%) on the main outcomes. The general linear model was used for meta-regression, with adjustment for multiple comparisons by using the Bonferroni method and weighting by study sample size.
Systematic Review
We used 177 publications that met inclusion and exclusion criteria for data extraction and final analyses (Figure 1; Technical Appendix Table 1). The studies were diverse in terms of design, data collection, and reporting approaches. We identified 20 case series (8 from South-East Asia region, 8 from the Western Pacific region, and 4 from Europe) and 21 cross-sectional studies (9 SouthEast Asia, 8 Western Pacific, and 4 Europe). Five articles about 3 outbreaks (in Sichuan and Jiangsu, China; and Phayao, Thailand) were classified as outbreak investigation reports. The only prospective case–control study was conducted in Vietnam (Table 1).
Epidemiology
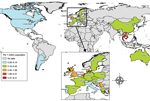
By the end of 2012, a total of 1,584 cases had been reported in the literature (including 189 probable cases identified in 3 outbreaks), mainly from Thailand (36%), Vietnam (30%), and China (22%). More than half (53%) were in the Western Pacific region; 36% were in the South East Asia region, 10.5% in the European region, and 0.5% in the Americas. The highest cumulative prevalence rate was in Thailand (8.21 cases/million population), followed by Vietnam (5.40) and the Netherlands (2.52) (country population data for 2008–2012 by World Bank [20]) (Figure 2).
The pooled mean age of the patients was 51.4 years, and 76.6% were men (Table 2). All case-patients were adults, except 1 female infant reported in Thailand (21). The pooled proportion of case-patients with occupational exposure was 38.1% (95% CI 24.4%–53.9%); this proportion was higher for industrialized countries than for other countries (83.8% [95% CI 73.4%–90.7%] for the United Kingdom, Netherlands, and Japan together). Recent contact with pigs or pork was reported for 15.5% of single cases but for 33.9% (95% CI 21.1%–49.5%) in the meta-analysis. History of eating meals containing pork was reported mainly in Asia (Thailand and Vietnam); the pooled estimate was 37.3% (95% CI 20.2%–58.3%). For Thailand only, the proportion was 55.8% (95% CI 33.7%–75.9%). In other countries, only 1 patient in France was reported eating artisanal dry sausage (22), and 1 patient in the United States ate raw pork while traveling in the Philippines (23) before the infection.
Skin injury was shown for one fourth of case-patients, and alcohol use was evident in approximately one third of case-patients. However, a case–control study in Vietnam did not identify alcohol use as an independent risk factor after adjustment for other risk factors and confounders (6). The most commonly reported preexisting condition was diabetes. Other conditions included underlying heart disease, hypertension, cirrhosis, and cancer (Technical Appendix Table 3). Smoking was mentioned in 5.2% of patients in the single-case dataset.
Microbiological Diagnosis
Blood and/or cerebrospinal fluid culture were the most common reported diagnostic methods among the case reports (Technical Appendix Table 4). Molecular diagnosis was more common in the large studies (11 studies) than in case reports. The most prevalent strain was serotype 2 (86.5%), followed by serotype 14 (2.3%), and serotype 1 (0.6%) of all 1,156 patients with serotype information mentioned in the articles. Serotypes 4, 5, 16, and 24 also were reported (1 patient per serotype).
Misdiagnosis of S. suis infection was not uncommon, either by conventional biochemical tests or commercial identification systems. The bacteria were often reported as viridans streptococci in initial cultures. In fact, up to 70% of all viridans streptococci cases in Thailand were confirmed as S. suis infections in the follow-up investigations (24). A total of 62.5% of S. suis–infected patients in another study in Thailand (25) and 20% in a study inthe Netherlands (10) were initially reported to be infected with viridans streptococci. Misidentification of the infectious agent as S. bovis (2 patients), S. pneumoniae (1 patient), and S. faecalis (1 patient) also was reported in the Netherlands series. Tsai et al. (26) showed large variations between the 2 commercial systems (Phoenix Identification System, Beckon Dickinson, Sparks, MD, USA; and Vitek II GPI Card, bioMérieux Vitek, Hazelwood, MO, USA), and misidentification of S. suis as S. acidominimus was common when the Phoenix system was used.
S. suis is mostly sensitive to penicillin; resistance was reported in only 2 patients (21,27). After cessation of antimicrobial drug treatment, infection relapsed in a small proportion of patients, including those for whom the organism tested highly sensitive to penicillin (28,29). The pooled relapse rate was 4.4% (Table 3).
Clinical Syndromes
Meningitis was the most common clinical syndrome (pooled rate 68.0% [95% CI 58.9%–75.8%]), followed by sepsis (25.0% [95% CI 20.5%–30.2%]), arthritis (12.9% [95% CI 6.0%–25.6%]), endocarditis (12.4% [95% CI 6.7%–21.9%]), and endophthalmitis (4.6% [95% CI 2.8%–7.4%]) (Table 3). Toxic shock syndrome also was reported as a distinct severe clinical feature at high rates in 2 outbreaks in China (64.0% and 28.9% of patients) (2,30) and in Thailand (37.7%) (24) but at a rate of only 2.9% among the case reports.
We found evidence of publication bias in the meta-analysis of meningitis rates (Figure 3) (significant Egger’s test result). The adjusted rate, based on the Duvall and Tweedie trim and fill method, was 56.0% (95% CI 45.2%–66.2%). Our meta-regression analysis showed that meningitis rate was significantly associated with country of publication, study design, and data collection approach (Technical Appendix Table 5), although only country of publication remained significant in a multivariable model. All patients in the 4 studies conducted in Vietnam had meningitis. When we excluded these studies, the pooled rate of meningitis was reduced to 59.4% (95% CI 51.1%–67.1%), and the adjusted value after we used the trim and fill method was 54.8% (95% CI 46.0%–63.4%). In contrast, if we excluded the 2 outbreak investigations in China, because sepsis dominated these outbreaks, the pooled meningitis rate increased slightly to 72.2% (95% CI 62.4%–80.2%).
Case-Fatality Rates
The pooled case-fatality rate (CFR) for S. suis–infected patients was 12.8% (95% CI 9.0%–18.0%) (Table 4). This rate varied by country; reported rates were lowest in Vietnam (Figure 4). However, country of publication was not significant in the bivariate meta-regression after adjustment for multiple comparisons (Technical Appendix Table 5). Instead, only meningitis rates remained significant in explaining between-study variations in CFR. Meningitis rates correlated negatively with CFRs among the included studies (Figure 5). Studies with meningitis rates <50% had significantly higher CFRs than did studies with meningitis rates >90% (mean CFR difference 20.3%, p = 0.001). The pooled CFR was 4.0% (95% CI 2.2%–7.0%), estimated for the studies in which all patients had meningitis (3,4,9,10,31–33), whereas the pooled rate for the other studies was 17.1% (95% CI 12.3%–23.4%). CFRs were higher for outbreaks than for nonoutbreaks (21.6% [95% CI 6.4%–52.5%] vs. 11.5% [95% CI 7.9%–16.7%]).
Clinical Outcomes
Among the survivors, hearing loss (pooled rate 39.1% [95% CI 31.0%–47.8%]) and vestibular dysfunction (22.7% [95% CI 15.6%–32.0%]) were the most common sequelae (Table 4). Reported rates for both sequelae varied widely, even within a country such as Thailand, (Technical Appendix Figures 1–4). Similar to CFRs, there was a marginally positive correlation between hearing loss and meningitis rates (p = 0.05) (Technical Appendix Table 5). The pooled hearing loss rate for studies in which all patients had meningitis was 55.3% (95% CI 36.2%–72.9%), compared with 34.0% (95% CI 26.0%–43.1%) for the remaining studies. For the vestibular dysfunction, none of the investigated study-level factors were significant. Among the Asian countries, the reported rate of vestibular sequelae was lowest in Vietnam (4.0%).
Limited information described how hearing loss and vestibular dysfunction were evaluated during and after infection. Eight of 25 large studies reporting hearing loss indicated whether hearing loss was permanent after hospital discharge. Only 4 described their follow-up processes; follow-up time ranged from 2 months to 30 months (4,8,28,31). On the basis of these limited data, we estimated a comparatively low median rate of recovery from hearing loss of 5.0% (range 0%–52.3%) and the pooled rate of 15.4% (95% CI 5.3%–37.3% (Table 4). Hearing loss might be mediated by adjunctive corticosteroid treatments, as was shown in a trial in Vietnam (34). Of the S. suis patients, 12.3% had deafness in at least 1 ear in the dexamethasone treatment group (n = 57), compared with 37.7% in the placebo group (n = 53).
We have updated estimates of the global prevalence, epidemiology, and clinical characteristics of S. suis infections in humans. After possible duplicates were removed, the total number of S. suis infections by 2012 was close to 1,600 cases, doubling the figure published in 2009 (12). Most of the increase comprised cases from Thailand and Vietnam, placing both countries in the highest disease prevalence stratum in the world. In contrast, only a few cases have been reported from the Americas, particularly the United States, the second largest producer of pigs worldwide (35). This low number might be attributable to the high industrialization of pig farming systems in the region. Nevertheless, we saw far more cases in Europe, a region where modern farming operations are presumably similarto those in the Americas. Other plausible explanations include the lower virulence of North American bacterial strains (36) or different slaughtering practices.
We counted only published cases; the actual number of cases would be considerably higher, particularly in areas to which S. suis is endemic, such as Asian countries with extensive pig rearing. The problem of underestimation is further exacerbated by the fact that S. suis infection is not a notifiable disease in many countries. In addition, lack of diagnostic capacities or limited disease awareness in local laboratories can result in undiagnosed or misdiagnosed cases.
Meningitis is the main syndrome in approximately two thirds of S. suis–infected patients, although this finding varied by country. The syndromic distribution of the reported cases may depend on study design and case ascertainment methods. All major studies in Vietnam ascertained S. suis cases from the population of patients with central nervous system diseases, which could lead to underrepresentation of S. suis patients with clinical syndromes other than meningitis. Only 1 patient without meningitis (diagnosed as spontaneous bacterial peritonitis with serotype 16 infection) has been reported in this country (37). Nevertheless, whether the existing strains infecting humans in Vietnam mainly cause meningitis remains unclear. In fact, lumbar puncture is performed regularly for all S. suis–infected patients, including those with severe sepsis, at a hospital for tropical diseases in Vietnam, and almost all had exhibited typical characteristics of bacterial meningitis in cerebrospinal fluid. On the other hand, meningitis might not be diagnosed or reported from other countries, therefore reducing the global S. suis meningitis estimate.
The difference in CFR between case-patients with meningitis and case-patients with severe sepsis has been documented in both outbreak and nonoutbreak situations in China and Thailand (1,2,24). Significantly more deaths were reported among S. suis patients with systemic infection, including hypotension, septic shock, multiorgan failure, and disseminated intravascular coagulation in these studies. In the Sichuan outbreak in 2005, the CFR reached 62% for patients classified as having streptococcal toxic shock syndrome (1). Several hypotheses have been suggested; however, the pathologic mechanisms underlying this high CFR remain to be elucidated (7,12). Regarding meningitis cases, the pooled CFR is lower than that for other common causes of adult bacterial meningitis, such as S. pneumoniae (19%–37%) (38) and Neisseria meningitidis (10%) (39). However, the rates of sequelae caused by S. suis tend to be higher than those caused by other agents reported in a recent meta-analysis (40).
We were unable to establish pooled risk estimates for different risk factors because of a lack of studies with appropriate designs. In the Netherlands, the annual risk for S. suis meningitis among abattoir workers and pig breeders was 1,500 times higher than that in the general population (10). In Vietnam, S. suis–infected patients were more likely to have eaten high-risk foods (odds ratio [OR] 4.38), to have pig-related occupations (OR 5.52), and to have pig exposure while having skin injuries (OR 15.96) than community controls (6). The lower proportions of patients with occupational exposure in Thailand and Vietnam than in Europe shown in our meta-analysis supports the hypothesis that other risk factors, including food consumption practices, may play a major role in the epidemiology of S. suis infection in Asia.
This review is not without limitations. The included studies were highly heterogeneous in quality and in the factors reported, which reduced the number of studies included in each meta-analysis. The summary values of the single-case dataset should be interpreted with caution because the patients in this merged “sample” were heterogeneously “recruited” from different populations, with different assessment protocols. In addition, the studies were mainly retrospective; data could have been easily missed on recall or by re-collecting from the existing data records. We were unable to assess the extent to which this misinformation could affect the overall estimates. However, data collection approach was not significantly associated with the main outcomes examined under this review in our meta-regression analyses.
This review helps to highlight areas in which additional research is needed. Geographic gaps obviously exist in the data on S. suis cases, especially in the pig rearing countries in the Americas, Eastern Europe, and Asia, such as Mexico and Brazil, Russia, and the Philippines, respectively. Second, much uncertainty remains in understanding sequelae of S. suis infection and recovery from these conditions over time. Careful prospective assessments of these debilitating outcomes and associated social and economic impacts are essential for understanding and reducing the effects of S. suis infection. More studies also are needed to assess the treatment effects of adjunctive corticosteroid on hearing loss or other neurologic sequelae.
The effects of S. suis infection are mainly in Asia; occupational exposure and eating possibly contaminated foods containing undercooked pig tissues are prime risk factors. Further research in Asia should focus on the factors pertinent to local risk for infection, including the practices of unsafe handling and consumption of pork. Prevention of human infections needs to be tailored for different risk groups, and studies are needed to assess the feasibility and effectiveness of those tailored programs. Additional work is needed to assess the fraction of S. suis cases that can be attributed to different risk factors (the population-attributable fraction) and the proportion of S. suis cases that might be preventable if specific risk factors were reduced.
Ms Huong is a DPhil student at the Nuffield Department of Medicine, University of Oxford, and is based at Oxford University Clinical Research Unit, Hanoi. Her primary research interests include epidemiologic and behavioral research on emerging infectious diseases in Asia; and the interface between animals and humans and their contribution to the occurrence and spread of diseases.
Acknowledgment
This study was supported in part by Global COE Program (2008–2012) and the Japan Initiative for Global Research Network on Infectious Diseases to K.H; by a “Grant-in-Aid for Scientific Research” from Nagasaki University to N.T. Huy (2007–2009); and by the Wellcome Trust Major Overseas Program and the Vietnam Initiative on Zoonotic Infections (2012–2016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–20 . DOIPubMedGoogle Scholar
- Hu X, Zhu F, Wng H, Chen S, Wang G, Sun J, Studies on human streptococcal infectious syndrome caused by infected pigs [in Chinese]. Zhonghua Yu Fang Yi XueZaZhi. 2000;34:150–2.
- Wertheim HF, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS ONE. 2009;4:e5973 . DOIPubMedGoogle Scholar
- Mai NT, Hoa NT, Nga TV. Linh le D, Chau TT, Sinh DX, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46:659–67.
- Hui AC, Ng KC, Tong PY, Mok V, Chow KM, Wu A, Bacterial meningitis in Hong Kong: 10-years' experience. Clin Neurol Neurosurg. 2005;107:366–70 . DOIPubMedGoogle Scholar
- Nghia HD, Tu le TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, et al. Risk factors of Streptococcus suis infection in Vietnam. A case–control study. PLoS ONE. 2011;6:e17604. PMID: 21408132
- Gottschal kM, Xu J, Lecours M-P, Grenier D, Fittipaldi N, Segura M. Streptococcus suis infections in humans: what is the prognosis for Western countries? (Part II). Clin Microbiol Newsl. 2010;32:97–102. DOIGoogle Scholar
- Navacharoen N, Chantharochavong V, Hanprasertpong C, Kangsanarak J, Lekagul S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol. 2009;123:857–62. DOIPubMedGoogle Scholar
- Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:868–76 .PubMedGoogle Scholar
- Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–7. DOIPubMedGoogle Scholar
- Walsh B, Williams AE, Satsangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Rev Med Microbiol. 1992;3:65–71.
- Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48:617–25. DOIPubMedGoogle Scholar
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–9. DOIPubMedGoogle Scholar
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. DOIPubMedGoogle Scholar
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. DOIPubMedGoogle Scholar
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to meta-analysis. Chichester (UK): Wiley; 2009.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. DOIPubMedGoogle Scholar
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: Higgins JPT, Green S, editors. The Cochrane Collaboration; 2011 [cited 2013 Apr 25]. http://www.cochrane-handbook.org
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. DOIPubMedGoogle Scholar
- World Bank. Data—population total. 2013 25/06/2013 [cited 2013 Jun 25]. http://data.worldbank.org/indicator/SP.POP.TOTL
- Vilaichone RK, Vilaichone W, Nunthapisud P, Wilde H. Streptococcus suis infection in Thailand. J Med Assoc Thai. 2002;85(Suppl 1):S109–17 .PubMedGoogle Scholar
- Bahloul H, Mofredj A, Mrabet A, Gineyt G, Rousselier P. Streptococcus suis meningitis after oral contamination? [in French]. Med Mal Infect. 2008;38:281–2. DOIPubMedGoogle Scholar
- Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. Streptococcus suis meningitis, United States. Emerg Infect Dis. 2008;14:183–5. DOIPubMedGoogle Scholar
- Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health. 2009;40:511–7 .PubMedGoogle Scholar
- Donsakul K, Dejthevaporn C, Witoonpanich R. Streptococcus suis infection: clinical features and diagnostic pitfalls. Southeast Asian J Trop Med Public Health. 2003;34:154–8 .PubMedGoogle Scholar
- Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000–2011. Diagn Microbiol Infect Dis. 2012;74:75–7. DOIPubMedGoogle Scholar
- Shneerson JM, Chattopadhyay B, Murphy MF, Fawcett IW. Permanent perceptive deafness due to Streptococcus suis type II infection. J Laryngol Otol. 1980;94:425–7. DOIPubMedGoogle Scholar
- Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM. 1995;88:39–47 .PubMedGoogle Scholar
- Woo J, Li EK. Streptococcus suis meningitis requires prolonged treatment with penicillin. Infection. 1987;15:129–30. DOIPubMedGoogle Scholar
- Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006;3:e151. DOIPubMedGoogle Scholar
- Rusmeechan S, Sribusara P. Streptococcus suis meningitis: the newest serious infectious disease. J Med Assoc Thai. 2008;91:654–8 .PubMedGoogle Scholar
- Nga TV, Nghia HD. Tu le TP, Diep TS, Mai NT, Chau TT, et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn Microbiol Infect Dis. 2011;70:461–7.
- Dragojlović J, Milosević B, Sasić N, Pelemis M, Sasić M. Streptococcus suis infection—clinical manifestations [in Serbian]. Med Pregl. 2005;58:236–9 . DOIPubMedGoogle Scholar
- Nguyen TH, Tran TH, Thwaites G, Ly VC, Dinh XS, Ho Dang TN, Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med. 2007;357:2431–40. DOIPubMedGoogle Scholar
- Wint W, Robinson T. Gridded livestock of the world 2007. Rome: Food and Agriculture Organization; 2007. p. 131.
- Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev. 2007;8:29–45. DOIPubMedGoogle Scholar
- Nghia HD, Hoa NT. Linh le D, Campbell J, Diep TS, Chau NV, et al. Human case of Streptococcus suis serotype 16 infection. Emerg Infect Dis. 2008;14:155–7.
- van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. DOIPubMedGoogle Scholar
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364:2016–25. DOIPubMedGoogle Scholar
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 20, Number 7—July 2014
| EID Search Options |
|---|
|
|
|
|
|
|


![Thumbnail of Meta-regression scatter plot showing the correlation between case-fatality rate and meningitis rate in a review of Streptococcus suis infection. The logit event rate was calculated for case-fatality rate as follows: logit event rate = ln[event rate/(1 − event rate)]. Each circle represents a study in the meta-analysis, and the size of the circle is proportional to study weighting. Studies with higher meningitis rates tended to report lower death rates.](/eid/images/13-1594-F5-tn.jpg)
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kenji Hirayama, Department of Immunogenetics, Institute of Tropical Medicine (NEKKEN), Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan
Top