Volume 21, Number 3—March 2015
CME ACTIVITY - Research
Epidemiology of Human Mycobacterium bovis Disease, California, USA, 2003–2011
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: February 11, 2015; Expiration date: February 11, 2016
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the epidemiology of Mycobacterium bovis infection in California, based on a retrospective review
• Identify risk factors for Mycobacterium bovis infection in California
• Distinguish the public health implications of the findings of this retrospective review regarding control of Mycobacterium bovis infection.
CME Editor
Claudia Chesley, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Claudia Chesley has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Mark Gallivan, MPH; Neha Shah, MD, MPH; and Jennifer Flood, MD, MPH, have disclosed no relevant financial relationships.
Abstract
We conducted a retrospective review of California tuberculosis (TB) registry and genotyping data to evaluate trends, analyze epidemiologic differences between adult and child case-patients with Mycobacterium bovis disease, and identify risk factors for M. bovis disease. The percentage of TB cases attributable to M. bovis increased from 3.4% (80/2,384) in 2003 to 5.4% (98/1,808) in 2011 (p = 0.002). All (6/6) child case-patients with M. bovis disease during 2010–2011 had >1 parent/guardian who was born in Mexico, compared with 38% (22/58) of child case-patients with M. tuberculosis disease (p = 0.005). Multivariate analysis of TB case-patients showed Hispanic ethnicity, extrapulmonary disease, diabetes, and immunosuppressive conditions, excluding HIV co-infection, were independently associated with M. bovis disease. Prevention efforts should focus on Hispanic binational families and adults with immunosuppressive conditions. Collection of additional risk factors in the national TB surveillance system and expansion of whole-genome sequencing should be considered.
Mycobacterium bovis, part of the Mycobacterium tuberculosis complex, is a zoonotic pathogen that can cause tuberculosis (TB) disease in a broad range of mammalian hosts (1). TB disease caused by M. bovis is clinically, radiographically, and pathologically indistinguishable from TB caused by M. tuberculosis (2). M. bovis transmission to humans most frequently occurs through consumption of unpasteurized, contaminated dairy products, but person-to-person transmission has been reported (3,4). The consumption of contaminated unpasteurized dairy products has been suggested as a major contributor to human M. bovis disease for several reasons: 1) the near absence of M. bovis disease among infants <12 months of age; 2) a high percentage of extrapulmonary disease, particularly abdominal disease, among patients with M. bovis disease; and 3) an association between positive interferon-γ release assay results and consumption of unpasteurized dairy products (5–11).
In the United States, 1%–2% of all human TB cases are attributable to M. bovis infection (7), but in certain geographic regions and communities, human M. bovis infection accounts for a much higher percentage of the cases. During 2001–2005, M. bovis accounted for nearly 10% of culture-positive TB isolates in San Diego, California, USA, including 54% of those from children (<15 years of age) and 8% of those from adults (>15 years of age) (8). Nearly all (97%) case-patients with M. bovis disease were among the Hispanic population, and 60% of those case-patients were born in Mexico (8). During 2001–2004, an investigation in New York, New York, USA, showed a high prevalence of TB caused by M. bovis among the Hispanic community. New York investigators reported that 57% of M. bovis case-patients were born in Mexico, and 83% of the interviewed case-patients consumed unpasteurized cheeses produced in Mexico while living in the United States (12).
The internationally recognized genotypic method for identifying M. bovis is spacer oligonucleotide typing (13). In 2004, the CDC (Centers for Disease Control and Prevention) Tuberculosis Genotyping Program (now called the National Tuberculosis Genotyping Service, http://www.cdc.gov/tb/publications/factsheets/statistics/genotyping.htm) began spoligotyping M. tuberculosis complex isolates from US patients with culture-positive TB (14). In California, the percentage of culture-positive isolates spoligotyped each year has gradually increased from 35% in 2004 to 92% in 2011. The incompleteness and variability (by geographic location) of spoligotype testing over this period exclude trend analysis and population-based M. bovis studies using this genotypic method. However, pyrazinamide monoresistance can serve as a proxy measure for M. bovis because M. bovis is intrinsically resistant to pyrazinamide but pyrazinamide monoresistance is rare among M. tuberculosis isolates (7,15,16). A recent national study on pyrazinamide resistance showed that 0.7% (196/27,428) M. tuberculosis isolates were pyrazinamide monoresistant (15). Since 2003, ≈97% of all culture-positive TB isolates in California have had initial (i.e., pretreatment) drug susceptibility testing for pyrazinamide, isoniazid, and rifampin.
M. bovis disease is of particular concern because of the high percentage of cases among children and because of its association with zoonotic and foodborne transmission, HIV co-infection, and poor treatment outcomes compared with M. tuberculosis disease (6,17–19). Further investigation into M. bovis disease is needed to understand the epidemiology of cases among children and adults, its association with immunosuppressive conditions, and the association of those conditions with treatment outcomes. We conducted a retrospective review of California TB surveillance data to evaluate trends for TB cases attributable to M. bovis, evaluate epidemiologic differences between M. bovis TB cases in adults and children, and identify risk factors associated with M. bovis disease compared with M. tuberculosis disease. We also conducted an evaluation of the accuracy of pyrazinamide monoresistance as a proxy measure for M. bovis disease by using surveillance and genotyping data.
The study population included all patients with culture-confirmed TB reported to the California TB registry during 2003–2011 and who had initial drug susceptibility testing results for isoniazid, rifampin, and pyrazinamide. Patients were classified as having M. bovis disease if the initial drug susceptibility results indicated resistance to pyrazinamide and susceptibility to isoniazid and rifampin. Sociodemographic, clinical, and treatment outcome information for all case-patients was abstracted from the TB registry. TB case registry data were matched to the California HIV/AIDS registry to identify HIV co-infection status. This analysis was conducted as part of the California Department of Public Health’s mandate to routinely collect and analyze surveillance data for public health purposes. The CDC (Atlanta, GA, USA) determined that the project was not human subject research and did not require approval by an institutional review board.
To compare the differences between child and adult populations, we stratified M. bovis case-patients by their age at the time TB was reported. The child population consisted of patients <15 years of age, and the adult population consisted of patients >15 years of age. To evaluate characteristics associated with TB disease caused by M. bovis compared with TB disease caused by M. tuberculosis, we conducted bivariate analysis with variables that were added to the national TB surveillance system in 2010. These variables include birth country of parents/guardians (for child case-patients), primary reason evaluated for TB disease, diabetes mellitus, and other immunosuppressive conditions. Immunosuppressive conditions, excluding HIV co-infection and diabetes mellitus, comprised end-stage renal disease, anti–tumor necrosis factor-α therapy–associated immunosuppression, solid organ transplant–associated immunosuppression, or other immunosuppressive condition as indicated by medical records or a health care provider. In addition, patient variables previously shown to be associated with TB disease caused by M. bovis (i.e., country of birth, race/ethnicity, age, HIV co-infection, site of disease, and death before treatment completion) were included in the bivariate analysis (7,19). Sociodemographic and clinical variables shown to be significantly associated with M. bovis disease at the bivariate level were put into a logistic regression model. The final logistic regression model was constructed by using the backward stepwise elimination procedure, removing predictors with p>0.05.
For TB cases during 2004–2011, the sensitivity and positive predictive value of the pyrazinamide monoresistance case definition were calculated by using spoligotyping data as the reference standard. The signature spoligotypes differentiating M. bovis and M. tuberculosis have been described previously in detail (20). Spoligotyping was conducted at the California Microbial Diseases Laboratory (Richmond, CA, USA), and the resulting data were entered into the TB Genotyping Information Management System (http://www.cdc.gov/tb/programs/genotyping/tbgims/default.htm). The TB registry and TB Genotyping Information Management System databases were merged by patients’ unique case numbers after spoligotyping data were deduplicated. Spoligotyping results indicating a genotype other than M. bovis or M. tuberculosis (including M. bovis bacillus Calmette-Guérin (BCG) strains) and isolates missing spoligotyping data or initial drug susceptibility testing results for pyrazinamide, isoniazid, or rifampin were excluded from analysis
Analyses were conducted by using SAS version 9.3 (SAS Institute, Cary, NC, USA). Separate Poisson regression models were used to identify temporal trends in the incidence of TB caused by M. tuberculosis and M. bovis. Year of TB case report was used as the explanatory variable, TB case number as the dependent variable, and state population size as the offset variable. Population denominators were obtained from the US Census Bureau’s current population survey (21). Trends of the annual percentage of TB cases attributable to M. bovis were examined by using the Cochran-Armitage trend test. Epidemiologic differences between M. bovis and M. tuberculosis were compared by using the χ2 test or Fisher exact test. Differences in median time to completion of therapy from start of therapy were analyzed by using the Wilcoxon rank-sum test. Trends and differences in disease characteristics were considered statistically significant if p<0.05 (2-sided).
Burden and Trends
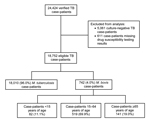
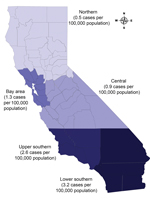
During 2003–2011, a total of 24,424 verified TB cases were reported in California. Of these, 5,061 (21.0%) culture-negative cases were excluded (Figure 1). Approximately 3.0% (611/19,363) of culture-positive cases were excluded because results of initial drug susceptibility testing for isoniazid, rifampin, or pyrazinamide were absent. M. bovis was identified by pyrazinamide monoresistance in 4.0% (742/18,752) of all eligible TB cases. M. bovis accounted for 22.0% (82/379) of culture-positive TB cases in children, 3.9% (519/13,397) of cases in adults 15–64 years of age, and 2.8% (141/4,976) of cases in adults >65 years of age. A total of 163 children <12 months of age had TB disease, and among those with culture-positive cases (81 children), none had M. bovis disease. The highest cumulative incidence of human M. bovis disease occurred in lower southern California (Figure 2).
During 2003–2011, TB incidence attributable to M. tuberculosis declined significantly (p<0.0001), but TB incidence attributable to M. bovis did not change (p = 0.92). The annual percentage of TB cases attributable to M. bovis among all age groups increased from 3.4% (80/2,384) in 2003 to 5.4% (98/1,808) in 2011 (p = 0.002; Figure 3). The annual percentage of TB cases attributable to M. bovis among the child population did not change significantly (p = 0.15), but the percentage among adults increased from 3.0% to 5.5% (p<0.001).
Epidemiology of M. bovis Disease in Children versus Adults
Several differences were observed between child and adult case-patients with M. bovis disease (Table 1). Child case-patients were more likely to be Hispanic and US-born and to have extrapulmonary disease. All M. bovis case-patients with concurrent HIV co-infection were adults. During 2003–2011, a total of 11.4% (80/699) of all case-patients with M. bovis disease died before treatment was completed. Among children, 1.2% (1/82) died before treatment was completed, compared with 12.8% (79/617) of adults (p = 0.002). Among case-patients who completed treatment, the median time to completion did not differ significantly between children and adults.
M. bovis Epidemiology versus M. tuberculosis Epidemiology
Bivariate analysis showed that the following patient characteristics were associated with TB caused by M. bovis but not by TB caused by M. tuberculosis: Hispanic ethnicity, birth in Mexico, incidental laboratory result as primary reason for TB evaluation, HIV co-infection, presence of extrapulmonary TB disease, and diabetes and other immunosuppressive conditions. Results were similar when we repeated the bivariate analysis using the spoligotyping method to identify M. bovis disease. A higher percentage of M. bovis– than M. tuberculosis–infected patients had diabetes; however, this association was no longer significant (p = 0.08) because the study population was smaller. The association between M. bovis disease and other immunosuppressive conditions remained significant (p = 0.002).
During 2010–2011, case-patients with M. bovis disease were more likely than those with M. tuberculosis disease to die before treatment completion (15.8% vs. 8.6%, p = 0.006) (Table 2). Among case-patients who died before treatment completion, those with M. bovis disease were more likely than those with M. tuberculosis disease to have had >1 concurrent immunosuppressive condition (73% vs. 53%, p = 0.05). Of the 26 M. bovis case-patients who died before treatment completion, 19 (73%) had >1 concurrent immunosuppressive condition: diabetes and end-stage renal disease (n = 6), diabetes (n = 5), HIV co-infection (n = 1), end-stage renal disease (n = 1), post–organ transplantation–associated immunosuppression (n = 1), end-stage renal disease and post-organ transplantation–associated immunosuppression (n = 1), or any other immunosuppressive condition (n = 4).
Subanalysis of child case-patients during 2010–2011 showed that 100% (6/6) of children with M. bovis disease had >1 parent/guardian who had been born in Mexico, compared with 38% (22/58) of children with M. tuberculosis disease (p = 0.005). In addition, 66% (4/6) of the children with M. bovis disease were born in the United States and 33% (2/6) were born in Mexico, compared with 93.1% (54/58) and 1.7% (1/58), respectively, of the children with M. tuberculosis disease (p = 0.02). Moreover, 66% (4/6) of the children with M. bovis disease had lived in Mexico for >2 months, compared with 3% (2/58) of the children with M. tuberculosis disease (p<0.001).
Multivariate analysis showed that Hispanic ethnicity (compared with non-Hispanic white), extrapulmonary disease, and diabetes and other immunosuppressive conditions were independently associated with M. bovis disease compared with M. tuberculosis disease (Table 3). Birth in a country other than the United States, excluding Mexico, was negatively associated with M. bovis disease compared with M. tuberculosis disease. Birth in Mexico was not independently associated with M. bovis disease (adjusted odds ratio 1.1, 95% CI 0.7–1.8).
Evaluation of the Pyrazinamide Monoresistance M. bovis Definition
Among all isolates with spoligotype and drug resistance data available during 2004–2011, the pyrazinamide monoresistance case definition had a sensitivity of 92% (95% CI 90%–95%) and positive predictive value of 82% (95% CI 79%–86%) for M. bovis. Among the Hispanic population, the sensitivity and positive predictive value of the pyrazinamide monoresistance definition was 94% (95% CI 92%–97%) and 96% (95% CI 94%–98%), respectively. When the analysis was restricted to the non-Hispanic Asian population, the sensitivity and positive predictive value dropped to 45% (95% CI 16%–75%) and 8% (95% CI 1%–14%), respectively. In the non-Hispanic Asian population, 92% (61/66) of pyrazinamide-monoresistant isolates were genotyped as M. tuberculosis.
In this large population-based epidemiologic study of M. bovis disease in California, we observed an increase in the annual percentage of TB cases attributable to M. bovis from 2003 through 2011. M. bovis disease accounted for nearly 25% of culture-positive TB cases in children. Patients with M. bovis disease were more likely than those with M. tuberculosis disease to die during treatment, and most deaths were among adults with concurrent immunosuppressive conditions. Hispanic ethnicity (compared with non-Hispanic white), extrapulmonary disease, and diabetes and other concurrent immunosuppressive conditions (excluding HIV co-infection) were independently associated with M. bovis disease compared with M. tuberculosis disease.
During 2010–2011, all (6/6) child case-patients with M. bovis disease had >1 parent/guardian who had been born in Mexico, and 4 of the 6 children had lived in Mexico for >2 months. These results, in conjunction with the a high percentage of extrapulmonary disease among the case-patients and the lack of M. bovis disease among children <12 months of age, are consistent with findings in previous epidemiologic studies that suggested that human M. bovis disease in the United States often results from consumption of unpasteurized dairy products originating from foreign countries, including Mexico (6,12,22,23). National pasteurization requirements, strict regulations on the importation of dairy cattle from Mexico into the United States, and an effective US bovine TB eradication program have substantially reduced M. bovis infection in US-born cattle (22,24). In the past century, the prevalence of M. bovis disease in US dairy herds has decreased from ≈5.0% to <0.001% (25). Furthermore, M. bovis isolates from humans in San Diego have been found to be genetically related to M. bovis strains from cattle in Mexico (22). Expansion of M. bovis disease surveillance and genotyping to include whole-genome sequencing may add discriminatory power beyond traditional genotyping and help to identify the source and route of transmission of M. bovis (26). Sharing of whole-genome sequencing data between countries and different health agencies may enhance future national and international prevention interventions.
Despite the relatively high percentage of extrapulmonary disease, 57% of M. bovis case-patients in our study had pulmonary involvement; this percentage is consistent with findings in other studies in the United States (7,8). Person-to-person transmission of M. bovis is considered infrequent, but the magnitude of such transmission has not been precisely quantified (27). Our findings are consistent with those from past research, which has shown that case-patients with M. bovis and M. tuberculosis pulmonary disease do not differ significantly in 2 of the key indicators of infectivity: presence of lung cavitation on chest radiographs and presence of acid-fast bacilli in sputum smears (6,7,28). In addition, previous research from pulmonary TB contact investigations showed that TB infection conversion rates among contacts did not differ significantly by mycobacterial species of the source case, suggesting that M. bovis is equally as transmissible as M. tuberculosis (28). However, current TB contact investigation guidelines do not include risk factors for M. bovis transmission. Although it is recommended to prioritize immunocompromised contacts of M. tuberculosis patients for evaluation, our data suggest that it may be even more important to prioritize immunocompromised contacts of M. bovis patients during contact investigations.
In our evaluation of the definition of pyrazinamide monoresistance, we found that isolates genotyped as M. tuberculosis may have been misclassified as M. bovis because of the pyrazinamide monoresistance definition. Misclassifications occurred most notably among the non-Hispanic Asian patients and might be explained by host, environmental (e.g., regional programmatic differences in TB treatment), and bacterial characteristics. From a recent national multivariate analysis of pyrazinamide resistance, Kurbatova et al. (15) suggested that bacterial lineage, not host characteristics, was the primary association between pyrazinamide monoresistance and M. tuberculosis disease.
Despite the possible definition-associated overestimation of M. bovis disease, the results from our study may still underestimate the true burden of M. bovis disease in California. Cases in children may be underestimated in the study population because sputum or gastric aspirate specimens are not consistently obtained from young children (29). In addition, M. bovis disease may be present in case-patients in the culture-negative subpopulation. We found case-patients with culture-negative TB to be similar to case-patients with culture-confirmed M. bovis disease with respect to age, extrapulmonary disease, and Hispanic ethnicity (data not shown).
Given the limitations of traditional genotyping and surveillance, we could not assess whether the increase in the number and percentage of adult M. bovis case-patients was to the result of recently acquired TB or reactivation of a previous infection. In addition, analysis of the new variables that were added to the national TB surveillance system in 2010 was hindered by the small number of child case-patients with M. bovis disease during 2010–2011. Also, because we did not conduct a medical chart review, we could not determine the cause of death among TB case-patients who died before the completion of TB treatment. Because of limitations in the national TB surveillance report form, we were unable to assess several possible relevant risk factors, including consumption of unpasteurized dairy products and the potential protective effect of the BCG vaccine against M. bovis disease. Although there is little information in the literature on the efficacy of the BCG vaccine in protecting against human M. bovis disease, the vaccine is notably protective against extrapulmonary disease and childhood TB disease, both of which are characteristic of M. bovis disease (30–33).
In summary, human M. bovis disease incidence has not declined in California, and the percentage of TB cases attributable to M. bovis has increased, exceeding the overall average for the United States. In California, there are ongoing interventions designed to limit the demand for and distribution of unpasteurized and contaminated dairy products, which are associated with M. bovis disease and other foodborne diseases (23). Elimination of human M. bovis disease in California likely requires further implementation of programs to reduce M. bovis contamination of dairy products in countries that have bovine TB, including pasteurization and test and cull interventions. In the interim, actions in the United States can help facilitate this effort. For example, M. bovis genotyping surveillance could be expanded and key risk elements (e.g., consumption of unpasteurized dairy products) could be captured in the national TB surveillance system to help determine M. bovis transmission routes. In addition, current TB contact investigation guidelines should be changed to include risk factors for M. bovis transmission. Last, future education efforts to prevent acquisition of M. bovis should focus on Hispanic binational families and adults with concurrent immunosuppressive conditions, including diabetes.
Mr. Gallivan is an epidemiologist in the Immunization Branch, California Department of Public Health. His research interests include TB infection treatment strategies, public health practice, and vaccine-preventable disease epidemiology.
Acknowledgments
We acknowledge the efforts of local health departments and laboratories in California who collected and reported the data used in this study.
This project was supported in part by the appointment of M.G. to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and by funding from CDC (cooperative agreement no. 1U38HM000414).
References
- LoBue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis. 2010;14:1075–8.PubMedGoogle Scholar
- Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb). 2001;81:71–7 and. DOIPubMedGoogle Scholar
- Sunder S, Lanotte P, Godreuil S, Martin C, Boschiroli ML, Besnier JM. Human-to-human transmission of tuberculosis caused by Mycobacterium bovis in immunocompetent patients. J Clin Microbiol. 2009;47:1249–51 . DOIPubMedGoogle Scholar
- Yates MD, Grange JM. Incidence and nature of human tuberculosis due to bovine tubercle bacilli in South-East England: 1977–1987. Epidemiol Infect. 1988;101:225–9. DOIPubMedGoogle Scholar
- Majoor CJ, Magis-Escurra C, van Ingen J, Boeree MJ, van Soolingen D. Epidemiology of Mycobacterium bovis disease in humans, the Netherlands, 1993−2007. Emerg Infect Dis. 2011;17:457–63. DOIPubMedGoogle Scholar
- LoBue PA, Betacourt W, Peter C, Moser KS. Epidemiology of Mycobacterium bovis disease in San Diego County, 1994–2000. Int J Tuberc Lung Dis. 2003;7:180–5 .PubMedGoogle Scholar
- Hlavsa MC, Moonan PK, Cowan LS, Navin TR, Kammerer JS, Morlock GP, Human tuberculosis due to Mycobacterium bovis in the United States, 1995–2005. Clin Infect Dis. 2008;47:168–75. DOIPubMedGoogle Scholar
- Rodwell TC, Moore M, Moser KS, Brodine SK, Strathdee SA. Tuberculosis from Mycobacterium bovis in binational communities, United States. Emerg Infect Dis. 2008;14:909–16. DOIPubMedGoogle Scholar
- Dankner WM, Davis CE. Mycobacterium bovis as a significant cause of tuberculosis in children residing along the United States–Mexico border in the Baja California region. Pediatrics. 2000;105:E79. DOIPubMedGoogle Scholar
- Garfein RS, Burgos JL, Rodriquez-Lainz A, Brodine S, Pietrucha A, Rondinelli A, Latent tuberculosis infection in a migrant agricultural community in Baja California, Mexico. J Immigr Minor Health. 2011;13:940–7. DOIPubMedGoogle Scholar
- Besser RE, Pakiz B, Schulte JM, Alvarado S, Zell ER, Kenyon TA, Risk factors for positive Mantoux tuberculin skin tests in children in San Diego, California: evidence for boosting and possible foodborne transmission. Pediatrics. 2001;108:305–10. DOIPubMedGoogle Scholar
- Center for Disease Control and Prevention. Human tuberculosis caused by Mycobacterium bovis—New York City, 2001–2004. MMWR Morb Mortal Wkly Rep. 2005;54:605–8 .PubMedGoogle Scholar
- Ramos DF, Tavares L, Almeida da Silva PE. Molecular typing of Mycobacterium bovis isolates: a review. Braz J Microbiol. 2014;45:365–72. DOIPubMedGoogle Scholar
- Ohkusa Y, Shigematsu M, Taniguchi K, Okabe N. Notice to readers: new CDC program for rapid genotyping of Mycobacterium tuberculosis isolates. MMWR Morb Mortal Wkly Rep. 2005;54(Suppl):47–52.PubMedGoogle Scholar
- Kurbatova EV, Cavanaugh JS, Dalton T, Click S, Cegielski JP. Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999–2009. Clin Infect Dis. 2013;57:1081–93. DOIPubMedGoogle Scholar
- de Jong BC, Onipede A, Pym AS, Gagneux S, Aga RS, DeRiemer K, Does resistance to pyrazinamide accurately indicate the presence of Mycobacterium bovis? J Clin Microbiol. 2005;43:3530–2. DOIPubMedGoogle Scholar
- Park D, Qin H, Jain S, Preziosi M, Minuto JJ, Mathews WC, Tuberculosis due to Mycobacterium bovis in patients coinfected with human immunodeficiency virus. Clin Infect Dis. 2010;51:1343–6. DOIPubMedGoogle Scholar
- Majoor CJ, Magis-Escurra C. van IJ, Boeree MJ, van SD. Epidemiology of Mycobacterium bovis disease in humans, the Netherlands, 1993–2007. Emerg Infect Dis. 2011;17:457–63. DOIPubMedGoogle Scholar
- LoBue PA, Moser KS. Treatment of Mycobacterium bovis infected tuberculosis patients: San Diego County, California, United States, 1994–2003. Int J Tuberc Lung Dis. 2005;9:333–8 .PubMedGoogle Scholar
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14 .PubMedGoogle Scholar
- United States Census Bureau. Population estimates; current estimate data [cited 2014 April 29]. http://www.census.gov/popest/data/index.htm
- Rodwell TC, Kapasi AJ, Moore M, Milian-Suazo F, Harris B, Guerrero LP, Tracing the origins of Mycobacterium bovis tuberculosis in humans in the USA to cattle in Mexico using spoligotyping. Int J Infect Dis. 2010;14(Suppl 3):e129–35. DOIPubMedGoogle Scholar
- Kinde H, Mikolon A, Rodriguez-Lainz A, Adams C, Walker RL, Cernek-Hoskins S, Recovery of salmonella, Listeria monocytogenes, and Mycobacterium bovis from cheese entering the United States through a noncommercial land port of entry. J Food Prot. 2007;70:47–52 .PubMedGoogle Scholar
- Code of Federal Regulations. Title 9—animals and animal products [9 CFR]. Animal and Plant Health Inspection Service, Department of Agriculture. Subchapter D—exportation and importation of animals (including poultry) and animal products. Part 93—importation of certain animals, birds, fish, and poultry, and certain animal, bird, and poultry products; requirements for means of conveyance and shipping containers [9 CFR 93]. Subpart D— ruminants; Mexico 9, 93.427–93.429; 2000 Jan 1 [cited 2014 April 29]. http://www.gpo.gov/fdsys/granule/CFR-2000-title9-vol1/CFR-2000-title9-vol1-sec93-427
- Palmer MV, Waters WR. Bovine tuberculosis and the establishment of an eradication program in the United States: role of veterinarians. Vet Med Int. 2011;2011:816345. PMID: 21647341
- Walker TM, Ip CL, Harrel RH, Evans JT, Kapatai G, Dedicoat MJ, Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13:137–46. DOIPubMedGoogle Scholar
- de Kantor IN, LoBue PA, Thoen CO. Human tuberculosis caused by Mycobacterium bovis in the United States, Latin America and the Caribbean. Int J Tuberc Lung Dis. 2010;14:1369–73 .PubMedGoogle Scholar
- LoBue PA, LeClair JJ, Moser KS. Contact investigation for cases of pulmonary Mycobacterium bovis. Int J Tuberc Lung Dis. 2004;8:868–72.PubMedGoogle Scholar
- Lighter J, Rigaud M. Diagnosing childhood tuberculosis: traditional and innovative modalities. Curr Probl Pediatr Adolesc Health Care. 2009;39:61–88. DOIPubMedGoogle Scholar
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. DOIPubMedGoogle Scholar
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. DOIPubMedGoogle Scholar
- García-Rodríguez JF, Álvarez-Díaz H, Lorenzo-García MV, Mariño-Callejo A, Fernandez-Rial Á, Sesma-Sánchez P. Extrapulmonary tuberculosis: epidemiology and risk factors. Enferm Infecc Microbiol Clin. 2011;29:502–9. DOIPubMedGoogle Scholar
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35 .PubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Epidemiology of Human Mycobacterium bovis Disease, California, USA, 2003–2011
CME Questions
1. You are consulting for an indigent care clinic in California regarding Mycobacterium bovis infection. According to the retrospective review by Gallivan and colleagues, which of the following statements about the epidemiology of M. bovis infection in California is correct?
A. The percentage of tuberculosis (TB) cases attributable to M. bovis decreased from 2003–2011
B. The percentage of TB cases caused by M. bovis was 5.4% in 2011
C. Among children younger than 15 years during 2010–2011, the percentage with at least 1 parent/guardian born in Mexico did not differ significantly between case patients with M. bovis and those with M. tuberculosis
D. One third of case patients with M. bovis had at least 1 parent/guardian born in Mexico
2. According to the retrospective review by Gallivan and colleagues, which of the following variables is most likely a risk factor for M. bovis infection in California?
A. African American race
B. Infection limited to the lungs
C. Diabetes
D. HIV/AIDS
3. According to the retrospective review by Gallivan and colleagues, which of the following statements about the public health implications of the findings regarding control of M. bovis infection is most likely correct?
A. Prevention efforts should target only California
B. Transmission is only via the respiratory route
C. Future educational efforts to prevent the acquisition of M. bovis infection should focus on non-Hispanic blacks
D. Expansion of M. bovis genotyping surveillance is recommended
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 21, Number 3—March 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Mark Gallivan, Immunization Branch, California Department of Public Health, 850 Marina Bay Parkway, Richmond, CA 94804-6403, USA
Top