Volume 29, Number 9—September 2023
Dispatch
Evaluating SARS-CoV-2 Saliva and Dried Blood Spot Surveillance Strategies in a Congregate Population
Abstract
The optimal approach to COVID-19 surveillance in congregate populations remains unclear. Our study at the US Naval Academy in Annapolis, Maryland, USA, assessed the concordance of antibody prevalence in longitudinally collected dried blood spots and saliva in a setting of frequent PCR-based testing. Our findings highlight the utility of salivary-based surveillance.
Congregate populations, including those in university and military settings, are at high risk for SARS-CoV-2 transmission because of crowding, frequent physical contact, and environmental contamination (1). Using self-collected saliva for surveillance may be a noninvasive alternative to serum and warrants further evaluation to guide population surveillance strategies.
Assessment of SARS-CoV-2 infection prevalence often is underestimated because of asymptomatic and paucisymptomatic infections that are not often captured by screening test strategies (2–4), but those infections contribute to high attack rates in congregate populations (5–9). This study evaluated the use of saliva to estimate the prevalence of SARS-CoV-2 infection among a congregate population of young and initially immunologically naive adults at the US Naval Academy (USNA) in Annapolis, Maryland, USA.
The Observational Seroepidemiologic Study of COVID-19 at the USNA (TOSCANA) study enrolled male and female midshipmen to estimate the SARS-CoV-2 attack rate and assess the concordance of seroprevalence between blood and saliva. All midshipmen at USNA (≈4,500) reside in a single dormitory. During the time of the study, nonpharmaceutical interventions included mask wearing, weekly PCR-based surveillance, and isolation of cases (Appendix). Pharmaceutical interventions included receipt of the Moderna (https://www.modernatx.com) SARS-CoV-2 mRNA vaccine in March 2021 (first dose) and April 2021 (second dose); >96% of all midshipmen had documented receipt of 2 doses.
We initiated the process of recruiting, enrolling, and acquiring consent of participants at the start of the academic year. After providing consent, participants completed the baseline questionnaire regarding demographic information, risk factors for acute respiratory infection, and previous infections or exposures to SARS-CoV-2. Paired self-collected saliva and dried blood spots were collected at enrollment (August 2020, visit 1 [V1]) and follow-up visits in December 2020 (V2), February 2021 (V3, saliva only), and April–May 2021 (V4) (Appendix Table).
Methods for dried blood spot (DBS) collection and testing has been described previously (10). We collected blood samples by using the Mitra Blood Collection Kit (Neoteryx, https://www.neoteryx.com) and tested them for SARS-CoV-2 reactive IgG by using an in-house multiplex microsphere-based immunoassay. The antigenic targets were a prefusion-stabilized SARS-CoV-2 spike glycoprotein ectodomain trimer and a nucleocapsid protein; we detected antigen-specific IgG levels by using a Bio-Plex 200 HTF multiplexing systems (Bio-Rad, https://www.bio-rad.com) and reported results as median fluorescence intensity (MFI).
We collected saliva samples by using an Oracol S14 collection device (Malvern Medical Developments, https://www.malmed.co.uk) and tested them as previously described (11). We tested samples for IgG binding to any of 7 SARS-CoV-2 antigen components (2 SARS-CoV-2 nucleocapsid proteins, 3 receptor-binding domain [RBD] proteins, and 2 spike proteins) by using a multiplex immunoassay. After background subtraction, we classified samples positive for RBD and nucleocapsid IgG as indicative of prior infection, whereas we classified samples positive for only RBD IgG as indicative of SARS-CoV-2 vaccination.
As part of routine clinical care, USNA’s Brigade Medical Clinic collected nasopharyngeal swab specimens from all returning midshipmen in August and throughout the school year when they visited the clinic with symptoms of respiratory illness. In addition, each week we randomly selected 15% of the asymptomatic midshipmen population for reverse transcription PCR (RT-PCR) screening; we also tested 100% of in-season varsity athletes each week. We excluded from weekly testing all participants who had confirmed positive SARS-CoV-2 infection during the preceding 90 days. We tested nasopharyngeal swab samples by using SARS-CoV-2 RT-PCR and made results accessible through electronic medical records.
We compared seroconversion rates with cumulative frequencies of molecularly confirmed infections. We calculated correlation coefficients for spike IgG and nucleocapsid IgG MFI in saliva and DBS. We used the Cohen kappa coefficient (κ) to measure concordance of saliva with DBS nucleocapsid IgG and spike IgG positivity and to measure concordance of PCR tests with seroconversions.
This study was approved by the Uniformed Services University Institutional Review Board under protocol IDCRP-129. All participants provided written informed consent.
In August 2020, a total of 104 midshipmen enrolled in the study; 64.4% were men, 92.3% were white, 8 (7.7%) reported COVID-19 exposure, and 11 (10.6%) reporting a COVID-19 diagnosis before arrival at USNA. At baseline, 17 (16%) participants showed evidence of SARS-CoV-2 infection based on spike IgG values in DBS.
Among the participants who were serologically negative for SARS-CoV-2 at enrollment, 18 seroconversions were detected in saliva, 19 were detected in DBS, and 19 were detected by PCR by the end of follow-up (Table 1); however, at V4 (postvaccination), additional cases were detected by DBS and saliva that were missed by PCR testing. One participant had a positive PCR result before a serologic result; the PCR test was conducted in August 2020, and the participant had no record of seroconversion through the end of the study.
By V4, 100% of remaining participants were spike IgG seropositive, and 49.1% of remaining participants with both DBS and saliva seroconverted to nucleocapsid IgG as evaluated by DBS (Table 2). Among participants with both DBS and saliva samples (n = 55), spike IgG results had an observed agreement of 0.85 and a κof 0.49 (95% CI 0.15–0.83) at V1. By V2 the observed agreement rose to 0.89 and κ to 0.61 (95% CI 0.32–0.91); by V4 the observed agreement reached 0.96. Nucleocapsid IgG results had an observed agreement of 0.95 and a κof 0.64 (95% CI 0.18–1.00) at V1 (Table 2). At V2 the observed agreement was 0.94 and κ was 0.69 (95% CI 0.36–1.00), and by V4 the observed agreement was 0.82 and κ was 0.64 (95% CI 0.43–0.84).
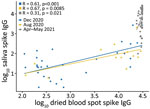
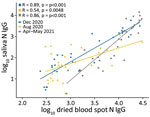
Spike IgG MFI in saliva and DBS were significantly correlated at all 3 timepoints (Figure 1); high spike IgG values at V4 were consistent with the participants receiving vaccinations in March–April 2021. Nucleocapsid IgG MFI in saliva and DBS also were significantly correlated at all 3 timepoints (Figure 2).
This study, conducted among a population of midshipmen at USNA in the first year of the COVID-19 pandemic, employed blood and saliva collection at multiple visits to evaluate the validity of salivary antibody surveillance. We observed concordance between DBS and saliva for the detection of spike and nucleocapsid IgG, and both biospecimen types were similar to RT-PCR for detection of cases. We noted that all vaccinees mounted a spike IgG response in DBS by V4, consistent with the known immunogenicity of these vaccines, but only 49.1% vaccinees had detectable nucleocapsid IgG at V4, indicating a substantive SARS-CoV-2 infection attack rate in the first half of 2021.
This assessment of SARS-CoV-2 detection in a congregate setting can help inform approaches for detection of SARS-CoV-2 in populations before and after vaccination. Prior evidence shows that PCR testing is an efficient method of infection control in congregate communities if administered regularly but that asymptomatic cases may still be undetected (12). These findings may apply to surveillance for other respiratory infections, such as influenza. A limitation to this study was the inability to directly match RT-PCR testing with blood and saliva collection, small sample size with paired samples, and loss to follow-up after the end of the academic year.
In summary, this assessment supports using saliva testing as a less invasive, more feasible surveillance method for monitoring changes in disease prevalence and susceptibility in large populations. Future directions include validation of alternative antibody targets, in both serum and saliva, which can discriminate antibody prevalence in the context of preexisting vaccination and postinfection hybrid immunity.
Dr. Andronescu is a postdoctoral fellow supporting research on SARS-CoV-2 with the Infectious Disease Clinical Research Program in the Department of Preventive Medicine and Biostatistics at the Uniformed Services University. Her other research interests include global health, control of infectious diseases, and surveillance in resource-limited settings.
Acknowledgments
This study (IDCRP-129) was conducted by the Infectious Disease Clinical Research Program, a Department of Defense program executed by the Uniformed Services University of the Health Sciences through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This work was supported by awards from the Defense Health Program (HU00012020067) and the National Institute of Allergy and Infectious Disease (HU00011920111). This project has been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, under an interagency agreement (Y1-AI-5072).
The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences; the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.; the Defense Health Agency; US Naval Academy; Departments of the Army, Navy, or Air Force; Department of Defense; or the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
C.C.B., T.H.B., J.M, E.D.L., A.K.S., and M.P.S. are service members or employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
References
- Diepstra K, Bullington BW, Premkumar L, Shook-Sa BE, Jones C, Pettifor A. SARS-CoV-2 seroprevalence: demographic and behavioral factors associated with seropositivity among college students in a university setting. J Adolesc Health. 2022;71:559–69. DOIPubMedGoogle Scholar
- Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54:12–6. DOIPubMedGoogle Scholar
- Ralli M, Morrone A, Arcangeli A, Ercoli L. Asymptomatic patients as a source of transmission of COVID-19 in homeless shelters. Int J Infect Dis. 2021;103:243–5. DOIPubMedGoogle Scholar
- Sah P, Fitzpatrick MC, Zimmer CF, Abdollahi E, Juden-Kelly L, Moghadas SM, et al. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc Natl Acad Sci U S A. 2021;118:
e2109229118 . DOIPubMedGoogle Scholar - Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, et al.; Public Health – Seattle & King County; CDC COVID-19 Investigation Team. Public Health–Seattle & King County; CDC COVID-19 Investigation Team. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–81. DOIPubMedGoogle Scholar
- Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic Transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–5. DOIPubMedGoogle Scholar
- Goldberg SA, Lennerz J, Klompas M, Mark E, Pierce VM, Thompson RW, et al. Presymptomatic transmission of severe acute respiratory syndrome coronavirus 2 among residents and staff at a skilled nursing facility: results of real-time polymerase chain reaction and serologic testing. Clin Infect Dis. 2021;72:686–9. DOIPubMedGoogle Scholar
- Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S, et al. CITIID-NIHR COVID-19 BioResource Collaboration. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife. 2020;9:9. DOIGoogle Scholar
- Han D, Li R, Han Y, Zhang R, Li J. COVID-19: Insight into the asymptomatic SARS-COV-2 infection and transmission. Int J Biol Sci. 2020;16:2803–11. DOIPubMedGoogle Scholar
- Epsi NJ, Richard SA, Laing ED, Fries AC, Millar E, Simons MP, et al.; EPICC COVID-19 Cohort Study Group. Clinical, immunological, and virological SARS-CoV-2 phenotypes in obese and nonobese military health system beneficiaries. J Infect Dis. 2021;224:1462–72. DOIPubMedGoogle Scholar
- Katz MJ, Heaney CD, Pisanic N, Smith L, Bigelow BF, Sheikh F, et al. Evaluating immunity to SARS-CoV-2 in nursing home residents using saliva IgG. J Am Geriatr Soc. 2022;70:659–68. DOIPubMedGoogle Scholar
- Hakre S, Lakhal-Naouar I, King DB, Burns JL, Jackson KN, Krauss SW, et al. Virological and serological assessment of US Army trainees isolated for coronavirus disease 2019. J Infect Dis. 2022;226:1743–52. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Mark P. Simons, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, 6720A Rockledge Dr, Bethesda, MD 20817, USA
Top