Volume 30, Number 3—March 2024
Research Letter
Subdural Empyema from Streptococcus suis Infection, South Korea
Abstract
In Jeju Island, South Korea, a patient who consumed raw pig products had subdural empyema, which led to meningitis, sepsis, and status epilepticus. We identified Streptococcus suis from blood and the subdural empyema. This case illustrates the importance of considering dietary habits in similar clinical assessments to prevent misdiagnosis.
Streptococcus suis is a zoonotic pathogen that affects pigs and humans when they handle pigs or eat undercooked pork products. Globally, an outbreak of infection occurred in China in 2005, and S. suis is a common cause of bacterial meningitis in Vietnam and Hong Kong (1,2). High-risk eating habits of ingesting raw or undercooked pork also have been reported in Thailand (2).
Although S. suis infection traditionally is associated with pig contact or consumption of undercooked pork, South Korea reported its first human infection in 2012, with subsequent cases not explicitly linked to pigs (3). Of note, consuming raw pork is rare in South Korea because of cultural taboos. In South Korea, the prevalence of S. suis infection was 12.6% among slaughtered pigs and 16.4% among diseased pigs; serotypes 2 and 14 were predominant in the Jeju area compared with other regions (4).
Common manifestations of S. suis infection are meningitis, endocarditis, septicemia, and arthritis but not subdural empyema (2). Subdural empyema is a rare but serious infection that causes a collection of pus between the dura and arachnoid layers of the meninges (5). We describe a case of subdural empyema caused by to S. suis infection after the consumption of raw pig products in Jeju Island, South Korea, where the pork industry has been an economic pillar for over 500 years.
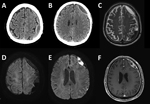
The patient, a 76-year-old man, visited the emergency department exhibiting dysarthria, neck stiffness, and right-sided weakness with motor grade III. He did not have hearing loss, a common symptom of human S. suis infection, or signs of increased intracranial pressure such as papilledema. His medical history included a fall 3 months prior and recent headache and dizziness. Initial brain computed tomography and magnetic resonance imaging showed chronic subdural hematoma (cSDH) with recurrent bleeding and an inflamed subdural sac (Figure 1). Concurrently, he exhibited septic symptoms, such as fever, hypotension, marked thrombocytopenia, and elevated inflammatory markers, necessitating immediate administration of antibiotics (vancomycin, ceftazidime, and metronidazole). Further studies showed that he did not have endocarditis, sinusitis, or otitis media (all possible causes of subdural empyema) (5). We drew blood cultures on admission day and on hospital days 4 and 7 and incubated them for >5 days. On hospital day 4, we detected S. suis from a blood culture. Subsequent inquiries into the patient’s dietary habits revealed recent consumption of Ae-Jeo-Hoe, a traditional dish from Jeju Island, made by slicing open the belly of a pregnant pig, finely chopping or grinding the fetus, and eating it raw with various seasonings. Consequently, we conducted further microbiologic investigations to rule out other conditions, such as severe fever with thrombocytopenia syndrome and cysticercosis, which all turned out negative.
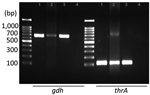
Upon confirmation of S. suis infection, treatment shifted to ceftriaxone. Results of blood cultures from days 4 and 7 were negative, but neurologic deficits persisted. On day 10, we evacuated a subdural empyema through left frontal and parietal burr hole trephinations. Intraoperatively, we identified a multiseptated pus-like tissue and a bloody subdural fluid. Despite the negative swab and fluid culture results, PCR confirmed S. suis gdh and thrA genes in the subdural empyema sample (Figure 2). We extracted total genomic DNA from blood-cultured bacteria and from the patient’s subdural hematoma by using the Solg Genomic DNA Prep Kit (SolGent, http://www.solgent.com) according to the manufacturer's instructions. We detected S. suis DNA by using the gdh-specific primers GCAGCGTATTCTGTCAAACG (forward) and CCATGGACAGATAAAGATGG (reverse) (6), and the thrA-specific primers GAAAATATGAAGAGCCATGTCG (forward) and GACAACGAACATAACAGAAACTTC (reverse) (7). In addition, we conducted next-generation whole-genome sequencing (Theragen Bio, https://www.theragenbio.com), which identified the isolate as serotype 2, which closely matched the genetic sequence of the ISU2614 strain (GenBank accession no. ASM1348816v1), known for its high virulence (8,9) (Appendix Figure).
On day 16, the patient had onset of convulsive status epilepticus potentially attributable to meningitis, which was managed by a continuous infusion of propofol. The patient was subsequently stabilized, and his neurologic symptoms improved.
This case is noteworthy because it represents a neurosurgical condition, specifically subdural empyema, which required surgery, associated with S. suis infection. In addition, severe conditions such as sepsis and status epilepticus after the infection underscore the lethality of this zoonotic pathogen.
The role of the patient’s cSDH caused by prior trauma warrants further discussion in the pathogenesis of the infection. The vascularized membrane of the cSDH may have served as a seeding bed for the hematogenous spread of the infection and development of the subdural empyema (10), suggesting increased susceptibility in patients with cSDH or older patients with a trauma history and emphasizing the need for prompt diagnosis and treatment.
This report highlights a unique correlation to consuming a traditional dish prepared from raw pig fetuses and underscores the importance of considering dietary habits in the clinical assessment. It also raises public health concerns about the potential risks associated with consuming raw or undercooked pork products and possible S. suis endemic in Jeju Island, where extensive pig rearing and consumption take place. Increasing disease awareness among clinicians and laboratories can prevent undiagnosed or misdiagnosed cases.
Dr. Choi is a neurosurgery resident at Seoul National University Hospital. His primary research interests include neurosurgical diseases and public health.
Acknowledgment
E.T.K. was supported by the National Research Foundation of Korea grant, funded by the Ministry of Science and Information and Communication Technology (grant no. 2021R1A2C1010313) and the Ministry of Education (grant no. RS2023-00270936), South Korea.
References
- Wertheim HF, Nghia HD, Taylor W, Schultsz C, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48:617–25. DOIPubMedGoogle Scholar
- Huong VTL, Ha N, Huy NT, Horby P, Nghia HDT, Thiem VD, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20:1105–14. DOIPubMedGoogle Scholar
- Kim J-G, Seong GM, Kim YR, Heo ST, Yoo JR. Streptococcus suis causes bacterial meningitis with hearing loss in patients without direct exposure to pigs in a regional pork industry territory. J Med Life Sci. 2023;20:43–7. DOIGoogle Scholar
- Oh SI, Jeon AB, Jung BY, Byun JW, Gottschalk M, Kim A, et al. Capsular serotypes, virulence-associated genes and antimicrobial susceptibility of Streptococcus suis isolates from pigs in Korea. J Vet Med Sci. 2017;79:780–7. DOIPubMedGoogle Scholar
- Baek SH, Choi SK, Ryu J, Lee SH. Subdural empyema treated by continuous irrigation and drainage catheter insertion in a young adult patient with hemiparesis: a case report. Nerve. 2017;3:85–8. DOIGoogle Scholar
- Okwumabua O, O’Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett. 2003;218:79–84. DOIPubMedGoogle Scholar
- Liu Z, Zheng H, Gottschalk M, Bai X, Lan R, Ji S, et al. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One. 2013;8:
e72070 . DOIPubMedGoogle Scholar - Fan H. Advances in pathogenesis of Streptococcus suis serotype 2. J Integr Agric. 2017;16:2834–47. DOIGoogle Scholar
- Nicholson TL, Waack U, Anderson TK, Bayles DO, Zaia SR, Goertz I, et al. Comparative virulence and genomic analysis of Streptococcus suis isolates. Front Microbiol. 2021;11:
620843 . DOIPubMedGoogle Scholar - Shapiro M, Walker M, Carroll KT, Levitt MR, Raz E, Nossek E, et al. Neuroanatomy of cranial dural vessels: implications for subdural hematoma embolization. J Neurointerv Surg. 2021;13:471–7. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: February 14, 2024
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eui Tae Kim or Jong-Kook Rhim, Jeju National University College of Medicine, Aran 13gil 15 (Ara-1 Dong) Jeju-si, Jeju Self-Governing Province, 63241, South Korea
Top