Volume 5, Number 1—February 1999
Research
Climatic and Environmental Patterns Associated with Hantavirus Pulmonary Syndrome, Four Corners Region, United States
Abstract
To investigate climatic, spatial, temporal, and environmental patterns associated with hantavirus pulmonary syndrome (HPS) cases in the Four Corners region, we collected exposure site data for HPS cases that occurred in 1993 to 1995. Cases clustered seasonally and temporally by biome type and geographic location, and exposure sites were most often found in pinyon-juniper woodlands, grasslands, and Great Basin desert scrub lands, at elevations of 1,800 m to 2,500 m. Environmental factors (e.g., the dramatic increase in precipitation associated with the 1992 to 1993 El Niño) may indirectly increase the risk for Sin Nombre virus exposure and therefore may be of value in designing disease prevention campaigns.
Relationships between environmental characteristics (i.e., climate, biome, elevation, habitat structure, and microhabitat), time, and hantavirus pulmonary syndrome (HPS) cases in the United States have not been systematically evaluated. We describe environmental factors associated with probable exposure sites for all diagnosed HPS cases in the Four Corners region (Arizona, New Mexico, Colorado, Utah) of the United States before 1996 and evaluate the strength of the associations between climate, time, and HPS cases.
All cases met the surveillance case definition for HPS, which requires the presence of clinically compatible symptoms and laboratory confirmation (1).
Fifty-nine sites in Arizona, New Mexico, Colorado, and Utah were identified as probable exposure sites for HPS cases (sites at which HPS patients were most likely infected with Sin Nombre virus (SNV), according to previously collected data from environmental assessments and individual patient questionnaires [2]) occurring from 1985 to 1995. Rodent trapping and testing data were also used to determine exposure sites, including those identified by linking hantavirus genome sequences between patients and rodents (3,4). Our inability to determine precisely where persons were exposed to HPS may have created a selection bias.
Climate analyses were limited to 52 probable exposure sites of HPS cases (n = 53 cases) (two case-patients were exposed to SNV at the same site); onset of illness was between 1993 and 1995. Climate data for probable exposure sites were collected from the nearest weather station monitored by the National Oceanic and Atmospheric Administration's Western Regional Climate Center in Reno, Nevada. Ten years (1986 to 1995) of monthly precipitation sums and monthly averages of daily ambient temperature were obtained for each station. To be included, sites had to be located within a 30-km radius and a 300-m elevation range from their closest corresponding weather station and without a mountain range between the exposure site and the identified weather station. Twelve sites did not meet these inclusion criteria, six were removed from precipitation analyses, and five were removed from temperature analyses because of missing weather data. Data from 24 weather stations were used for the precipitation (n = 34 exposure sites) and temperature analyses (n = 35 exposure sites). Four weather stations represented multiple exposure sites because they were the closest available weather stations. Twenty-two (85%) exposure sites included in the analyses were within 15 km (mean = 13.2 km, standard deviation [SD] = 7.9 km) and 150 m of elevation (mean = 78.0 m, SD = 79.2 m) of their corresponding weather station. Data were analyzed with the SPSS and Epi-Info statistical software programs (5,6).
The use of climatic data collected from weather stations as far as 30 km away from probable exposure sites, with as much as a 300-m difference in elevation, may have created an ascertainment bias. Weather patterns, however, generally cover large regions. In addition, a withdrawal bias may have been introduced by eliminating exposure sites and weather stations that did not meet the inclusion criteria.
Precipitation Analysis
Precipitation totals from the 24 identified weather stations for each of the 48 months during 1992 through 1995 were compared with the corresponding calendar month's mean precipitation total for 1986 to 1991. Each month's mean precipitation difference was plotted against the number of HPS cases with onset of symptoms during that month for cases between 1993 and 1995. The Wilcoxon matched-pairs signed rank test was used to test the statistical significance of precipitation during two periods of substantial departure from normal precipitation patterns (i.e., 6-year mean sum precipitation data for the same calendar months). These weather stations, representing the 1993 to 1995 cases, reported above average (p < 0.01) precipitation totals during December 1992 through March 1993 and below average (p < 0.01) precipitation totals during June 1993 through July 1993 (Figure 1).
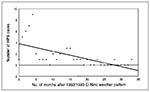
By using a Spearman's correlation, we found a negative correlation between the number of cases per month (onset date) and the number of months after the 1992 to 1993 El Niño weather pattern (rs = -0.70; p < 0.01) (Figure 2). For this portion of the precipitation analysis, the months of onset were used for all 53 cases between 1993 and 1995. The end of the 1992 to 1993 El Niño was described as the month (March 1993) in which the abnormally high precipitation totals for November 1992 through March 1993 returned to normal, on the basis of the data in Figure 1.
The 1993 outbreak of HPS in the Four Corners region followed a dramatic increase in precipitation associated with the 1992 to 1993 El Niño phenomenon and peaked in the middle of a drought. Because the 1992 to 1993 El Niño resulted in an abundance of rodent food resources (e.g., vegetation and insects) and a 20-fold rodent population increase over the previous year at the Sevilleta National Wildlife Refuge in central New Mexico, increased rainfall from El Niño was associated with the 1993 HPS outbreak (7). A similar pattern of above average rainfall followed by drought was observed preceding an outbreak of HPS in western Paraguay in 1995 to 1996 (8). Our study shows that the number of HPS cases per month in the Four Corners region during 1993 to 1995 was negatively correlated with the number of months after the 1992 to 1993 El Niño. The data suggest that the association between the 1992 to 1993 El Niño and the number of HPS cases in the Southwest may have lasted for as long as 2 years.
The association between El Niño and the HPS outbreak is probably complex. The above average precipitation during the winter and spring of 1992 to 1993 may have increased rodent populations and thereby increased the likelihood of more rodent-to-rodent contact, rodent-to-human contact, and viral transmission, thereby resulting in the large number of cases in 1993 and 1994. In addition, as rodent populations surpassed the carrying capacity of their local environments and precipitation plummeted, available food sources may have been depleted, resulting in rodent population stress. Increased stress likely increased rodent-to-rodent contact, as rodents competed for food and water, and increased rodent-to-human contact, as rodents moved into new, potentially less stressful environments, such as homes and peridomestic structures.
During 1995, no cases occurred in the original outbreak area near the Arizona-New Mexico border and in western Colorado, possibly because the effect of the 1992 to 1993 El Niño had dissipated. Preliminary data from longitudinal trapping studies in the Southwest suggest that the relative rodent densities decreased to normal levels in 1995 (J. Mills, T. Yates, pers. comm.). Hantavirus infection rates in rodents dramatically decreased at case sites in Arizona and Colorado 3 years after the outbreak (9).
Monthly ambient temperature means from the 24 identified weather stations for each of the 48 months during 1992 through 1995 were compared to the corresponding calendar month's ambient temperature mean for 1986 to 1991. The mean difference was plotted against the number of HPS case onsets per month for cases occurring between 1993 and 1995. With all 24 weather stations included, the Wilcoxon matched-pairs signed rank test was used to test the statistical significance of two periods with substantial departures from normal temperature patterns (i.e., 6-year mean daily temperature averages for the same calendar months): 1) November 1992 through December 1992 and 2) January 1993. The 24 weather stations reported below average (p < 0.01) temperatures during November 1992 through December 1992, which corresponded with the onset of El Niño (Figure 1). Conversely, the mean temperature for January 1993 was above average (p < 0.01).
While interpreting variations in precipitation totals is fairly straightforward, interpreting variations in ambient temperature is far more difficult. Whether the lower-than-average ambient temperature during November 1992 through December 1992 affected rodent population dynamics is unknown; however, the higher-than-average temperatures during January 1993 may have promoted rodent survival during what is normally the coldest month of the year. (Figure 1 indicates that the 24 weather stations reported the most substantial mean temperature extremes in 1995.)
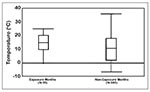
To examine the relationship between ambient temperature and month of exposure for HPS cases, we compared the distribution of daily ambient temperatures of probable month (defined as the month in which onset date minus 14 days [hypothesized mean incubation period] occurred) of exposure to daily ambient temperatures during the other 11 months of the same calendar year. Exposure months had higher ambient temperatures and a smaller temperature range than the other months in the same calendar year. The mean temperature for the probable exposure months was 15°C (SD = 7°C; range = 0°C - 24°C) (Figure 3), and the mean throughout the rest of the year was 11°C (SD = 9°C; range = -7°C - 36°C).
Spatial and environmental data were collected for 59 of the 64 known HPS cases that occurred before 1996 in the Four Corners region: Arizona (20 sites), New Mexico (25 sites), Colorado (7 sites), and Utah (7 sites). For five cases (in 1959, 1975, 1984, 1985, and 1993), the exposure site was not known. Habitat data (e.g., dominant biome type as described by Brown [10] within 200 m) were collected at each of the 59 probable exposure sites. Elevations were taken from U.S. Geological Service quadrant maps. All analyses were conducted with the SPSS and Epi-Info statistical software programs (5,6).
Spatial Analysis
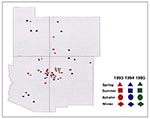
Figure 4, Figure 5, and Figure 6 display the spatial and temporal distribution of probable hantavirus exposure sites in the Four Corners region, from 1993 through 1995 (n = 52). In 1993, most case-patients were exposed in northeastern Arizona and northwestern New Mexico; in 1994, HPS case-patients were exposed in all four states, including two new areas, central Utah and southern Arizona; and in 1995, HPS case-patients were exposed only in a small geographic area near the border of southcentral Colorado and northcentral New Mexico (on the eastern slope of the Rocky Mountains).
From 1993 through 1995, HPS cases in the Four Corners region shifted geographically. The apparent spatial and temporal movement of HPS foci in this region mirrors the "focal nidality" exhibited by hemorrhagic fever with renal syndrome (HFRS), the Eurasian hantaviral manifestation (11-15). HFRS cases can occur sporadically throughout the year, but outbreaks occur seasonally (12,16). Seasonal prevalence varies by locality and meteorologic and climatic conditions that favor activity of rodents associated with viruses causing HFRS (12). Niklasson et al. (17) hypothesize that large outbreaks of HFRS occur in geographic "hot spots," which may depend on certain ecologic characteristics correlating with rodent populations, e.g., rodent habitats and environmental or meteorologic conditions (17). In the Four Corners states, seroprevalence of hantaviruses in rodents displays a similar focality (18). Our data suggest that HPS cases may have occurred in similar hot spots in this region between 1993 and 1995.
Temporal Analysis
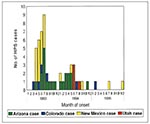
The 1993 to 1995 HPS onset months were unevenly distributed (Kolmogrov-Smirnov test, p = 0.01), displaying a spring-summer seasonality, as has been reported previously (2,3,15) (Figure 6). While cases occurred throughout all seasons, probable exposures occurred during the time of year when the monthly mean ambient temperature was 0°C to 24°C.
The seasonality of HPS cases varies by location, elevation, and biome (Figure 4, Figure 5, and Figure 7). The clustering of all four Sonoran Desert HPS cases during the late winter and early spring and the two montane conifer cases during the late spring and summer, when the mean temperatures for both biomes are mild (12°C - 21°C), are the best examples of this trend. Most grassland, Great Basin scrub, pinyon-juniper, and montane conifer cases occurred during spring and summer (Figure 7).
Environmental Analysis
Probable exposure sites occurred in seven biomes, most often in pinyon-juniper woodland, grassland, and Great Basin desert scrub biomes (33.9%, 28.8%, and 23.7%, respectively) (Table). Approximately 66% (39/59) of case-patients were exposed at elevations of 1,800 m to 2,500 m; none was exposed at elevations higher than 2,500 m (Table). This description may reflect the typical biomes in which the 1993 outbreak took place but also holds for cases in Utah and Colorado. Mills et al. (18) reported the same habitat and elevation for sites with the highest Peromyscus densities (as well as the highest rodent SNV antibody prevalence) in the Four Corners states. Unpublished case-control data (J. Cheek and R. Bryan) show that significantly more HPS cases occurred in Great Basin pinyon-juniper than in any other biome on the Navajo reservation in the Southwest; the data also show that HPS cases are more likely at higher elevations. Environmental conditions surrounding HPS exposure sites appear similar to those surrounding plague exposure sites on the Navajo reservation (K. Gage, R. Enscore, unpub. data).
| Biome type | Frequency (n = 59) | % |
|---|---|---|
| Sonoran Desert | 4 | 6.8 |
| Chihuahuan Desert | 1 | 1.7 |
| Great Basin Desert scrub | 14 | 23.7 |
| Great Basin and plains grassland | 17 | 28.8 |
| Great Basin pinyon-juniper | 20 | 33.9 |
| Riparian woodland | 1 | 1.7 |
| Montane conifer forest | 2 | 3.4 |
| Elevation range | ||
| 0 - 619 | 3 | 5.1 |
| 620 - 1,239 | 1 | 1.7 |
| 1,240 - 1,859 | 13 | 22.0 |
| 1,860 - 2,480 | 42 | 1.2 |
Probable exposure sites were most frequently found in rural areas, with five or fewer homes within a 1-km radius (64.4%); most exposure sites (88.1%) were found in areas with 25 or fewer homes. An almost equal number of exposure sites (44.1%) was more than 2 km away from a permanent water source as exposure sites (42.4%) within 1 km of a permanent water source. Overall, 104 plant species belonging to 72 genera were identified at exposure sites. The three most common dominant herbaceous species were Artemisia tridentata (big sagebrush), Gutierrezia sarothrae (snakeweed), and Bromus tectorum (downy chess grass); and the three most common dominant overstory and understory species were Juniperus monosperma (one-seed juniper), J. osteosperma (Utah juniper), and Pinus edulis (two-needle pinyon pine). For 21 of 59 sites with 5% slope or greater, more than twice as many had a northward aspect (9 of 21) than any other aspect (south = 4 of 21; east = 4 of 21; and west = 4 of 21).
While the habitat may have changed in minor ways between the time of exposure and data collection, no major habitat changes (e.g., fire, timber harvesting) were identified. Because these environmental findings are descriptive, caution should be used when drawing conclusions on the basis of these data.
Previous studies have examined behavior and environmental risk factors for the acquisition of HPS (19-22). Certain behavior and occupations may increase the likelihood of exposure to the excreta of infected rodents; however, the overall level of risk seems low (21,22). Environmental risk factors may be divided into endemic and epidemic. Endemic environmental risk factors are tied to the static habitat structure of a geographic area. For example, the habitat of the Four Corners region allows population levels of Peromyscus maniculatus great enough to maintain SNV transmission (18,23). Some level of risk will likely always exist for exposure to SNV in the region; however, the level seems low (9). Epidemic environmental risk factors, also tied to the static habitat structure of a geographic area (i.e., they can only occur in disease-endemic areas), are primarily dynamic events associated with large-scale environmental changes of limited duration. For example, the precipitation pattern during the 1992 to 1993 El Niño, associated with increased rodent populations in the Southwest (7) and consequently with the 1993 outbreak of HPS in the Four Corners region, greatly increased the level of risk for human SNV infection in the outbreak area. By 1995, rodent populations (J. Mills, T. Yates, pers. comm.) and the number of HPS cases in this area dramatically decreased, and the level of risk for human SNV infection may have returned to endemic levels in 1995. Early recognition of these endemic and epidemic environmental risk factors might allow public health agencies to predict where and when the next HPS outbreak may occur and thus to effectively target prevention efforts.
While this report does not address data related to cases after 1995, the 1997 to 1998 El Niño may not have displayed the same precipitation pattern in the Four Corners region as the 1992 to 1993 El Niño. Limited data from Arizona's primary weather station for northern Arizona indicate 4.70 cm of precipitation in December 1997—the 98-year mean (1899–1997) for December is 4.88 cm (Office of the State Climatologist—Arizona State University, pers. comm.). In December 1992, the same station reported 17.22 cm of precipitation (Office of the State Climatologist—Arizona State University, pers. comm.). Future studies should examine and compare the two precipitation events, their lasting effects on rodent populations, and the subsequent effects on hantavirus epidemiology.
David Engelthaler is a visiting fellow, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO. Mr. Engelthaler is involved in plague research. His primary research interest is pathogen-vector relationships.
Acknowledgments
We thank Rosalyn Curtis, Herman Shorty, Rusty Enscore, Dave Tibbs, Chuck Freeman, Benny Joe, Bobby Villines, Eric Faist, Charlie Irland, Tim Doyle, and Dorothy Miller for their assistance in data collection and Charles Calisher, T. Michael Fink, Nicole Rossi, Mira Leslie, and Thomas G. Engelthaler for their critical review and comments.
This study was supported in part by CDC cooperative agreements with Arizona, Colorado, New Mexico, and Utah.
References
- Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep. 1997;46(No.RR-10):16.
- Khan AS, Khabbaz RF, Armstrong LR, Holman RC, Bauer SP, Graber J, Hantavirus pulmonary syndrome: the first 100 U.S. cases. J Infect Dis. 1996;173:1297–303.PubMedGoogle Scholar
- Hjelle B, Torrez-Martinez N, Koster FT, Jay M, Ascher MS, Brown T, Epidemiologic linkage of rodent and human hantavirus genomic sequences in case investigations of hantavirus pulmonary syndrome. J Infect Dis. 1996;173:781–6.PubMedGoogle Scholar
- Nichol ST, Spiropoulou CF, Morozunov S, Rollin PE, Ksiazek TG, Feldman , . Genetic identification of a novel hantavirus associated with an outbreak of acute respiratory illness in the southwestern United States. Science. 1993;262:615–8. DOIGoogle Scholar
- SPSS for Windows [computer program]. Version 6.1. Chicago: SPSS, Inc.; 1994.
- Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, Epi Info [computer program]. Version 6. A word processing, database, and statistics program for epidemiology on microcomputers. Atlanta (GA): Centers for Disease Control and Prevention; 1994.
- Parmenter RR, Brunt JW, Moore DI, Ernest S. The hantavirus epidemic in the Southwest: rodent population dynamics and the implications for transmission of hantavirus-associated adult respiratory distress syndrome (HARDS) in the Four Corners region. Department of Biology, University of New Mexico, Albuquerque, New Mexico; 1993. Sevilleta Long-Term Ecological Research Program (LTER); Publication No.: 41.
- Williams RJ, Bryan RT, Mills JN, Palma RE, Vera I, De Velasquez F, An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg. 1997;57:274–82.PubMedGoogle Scholar
- Engelthaler DE, Levy CL, Fink TM, Tanda D, Davis T. Decrease in seroprevalence of antibodies to hantavirus in rodents from 1993-1994 hantavirus pulmonary syndrome cases. Am J Trop Med Hyg. 1998;58:737–8.PubMedGoogle Scholar
- Brown DE. Biotic communities: southwestern United States and northwestern Mexico. Salt Lake City. University of Utah Press. 1994;li:49–221.
- McKee KT Jr, LeDuc JW, Peters CJ. Hantaviruses. In: Belshe RB, editor. Textbook of human virology. 2nd ed. St Louis (MO): Mosby Year Book; 1991. li. 615-32.
- Mayer CF. Epidemic hemorrhagic fever of the Far East, or epidemic hemorrhagic nephroso-nephritis. Mil Surg. 1952;110:276–85.PubMedGoogle Scholar
- Gauld RL, Craig JP. Epidemiological pattern of localized outbreaks of epidemic hemorrhagic fever. Am J Hyg. 1954;59:32–8.PubMedGoogle Scholar
- Pon E, McKee KT Jr, Diniega BM, Merrell B, Corwin A, Ksiazek TG. Outbreak of hemorrhagic fever with renal syndrome among U.S. Marines in Korea. Am J Trop Med Hyg. 1990;42:612–9.PubMedGoogle Scholar
- Chapman LE, Khabbaz RF. Etiology and epidemiology of the Four Corners hantavirus outbreak. Infect Agents Dis. 1994;3:234–44.PubMedGoogle Scholar
- World Health Organization. Haemorrhagic fever with renal syndrome: memorandum from a WHO meeting. Bull World Health Organ. 1983;61:269–75.PubMedGoogle Scholar
- Niklasson B, Jonsson M, Widegren I, Persson K, LeDuc J. A study of nephropathia epidemica among military personnel in Sweden. Res Virol. 1992;143:211–4. DOIPubMedGoogle Scholar
- Mills JN, Ksiazek TG, Ellis BA, Rollin P, Nichol ST, Yates TL, Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities in the southwestern United States. Am J Trop Med Hyg. 1997;56:273–84.PubMedGoogle Scholar
- Zietz PS, Butler JC, Cheek JE, Samuel MC, Childs JE, Shands LA, A case-control study of hantavirus pulmonary syndrome during an outbreak in the southwestern United States. J Infect Dis. 1995;171:864–70.PubMedGoogle Scholar
- Childs JE, Krebs JW, Ksiazek TG, Maupin GO, Gage K, Rollin PE, A house-hold based, case-control study of environmental factors associated with hantavirus pulmonary syndrome in the southwestern United States. Am J Trop Med Hyg. 1995;52:393–7.PubMedGoogle Scholar
- Armstrong LR, Khabbaz RH, Childs JE, Rollin PE, Martin ML, Clarke M, Occupational exposure to hantavirus in mammalogists and rodent workers [abstract]. Am J Trop Med Hyg. 1994;51:94.
- Zietz PS, Graber JM, Voorhees RA, Kioski C, Shands LA, Ksiazek TG, Assessment of occupational risk for hantavirus infection in Arizona and New Mexico. J Occup Environ Med. 1997;39:463–7. DOIPubMedGoogle Scholar
- Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J Infect Dis. 1994;169:1271–80.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 5, Number 1—February 1999
| EID Search Options |
|---|
|
|
|
|
|
|