Volume 7, Number 3—June 2001
Dispatch
Borrelia lonestari DNA in Adult Amblyomma americanum Ticks, Alabama
Abstract
Polymerase chain reaction analysis of 204 Amblyomma americanum and 28 A. maculatum ticks collected in August 1999 near the homes of patients with southern tick-associated rash illness and in control areas in Choctaw County, Alabama, showed Borrelia lonestari flagellin gene sequence from two adult A. americanum. The presence of B. lonestari in A. americanum ticks from Alabama suggests that this suspected pathogen may be widespread in the southeastern United States.
Lyme disease is the most commonly reported vector-borne disease in the United States. In the northeastern, midwestern, and western coastal states, the pathogen Borrelia burgdorferi sensu lato is well established and is maintained by Ixodes scapularis and I. pacificus in a variety of rodent reservoirs.
In the southeastern states, where I. scapularis is widespread but is less commonly found infected with B. burgdorferi or attaching to humans (1,2), isolations from humans are uncommon (3). However, a clinical condition similar to Lyme disease, termed southern tick-associated rash illness (STARI), has been described in humans in the southeastern region of the United States associated with the bite of Amblyomma americanum ticks (1,4,5). Moreover, a new spirochete, B. lonestari, was described from A. americanum on the basis of polymerase chain reaction (PCR) amplification of the flagellin and 16s rRNA genes (6,7). Virtually identical sequences have been found in ticks from geographic regions as disparate as New Jersey and Texas (6), suggesting this organism is widely distributed. Likewise, Borrelia spirochetes have been detected in A. americanum and I. scapularis in Alabama (8,9).
As part of an epidemiologic investigation of a reported cluster of STARI cases in Choctaw County, Alabama, we collected both A. americanum and A. maculatum ticks adjacent to the houses of suspected patients and in control areas, and the B. lonestari flagellin gene sequence was amplified from DNA extracted from A. americanum.
Ticks were collected with drag cloths in areas around the homes of persons with suspected cases, as well as in control areas. Clinical cases were defined as illness characterized by acute onset of an annular, expanding erythema migrans-like rash at least 5 cm in diameter, when no alterative explanation for the rash can be found; and there is a history of tick bite at the rash site or potential exposure to ticks within 14 days before rash onset. Ticks were identified to species by using standard taxonomic keys.
DNA was isolated from Amblyomma sp. by using an extraction procedure reported previously (10). Briefly, individual ticks were frozen in liquid nitrogen, macerated between metal plates, and then homogenized by adding 1 mL of DNA STAT-60 (Tel-Test, Friendswood, TX). The tick homogenate was then incubated with chloroform for phase separation of DNA, which was precipitated with 100% isopropanol. To ensure PCR-quality DNA, all tick extracts were tested for the presence of tick mitochondrial DNA (11). Amblyomma DNA was then subjected to a nested PCR procedure for B. lonestari by using primers FlaLL/FlaRL, then FlaLS/FlaRS (6). Flagellin-positive samples were further analyzed by using OspA primers as a control for possible flagellin false-positive samples. Subsequent DNA sequencing of positive samples for Borrelia sequence identification was done with a Taq Dyedeoxy terminator cycle kit (Applied Biosystems, Foster City, CA) and run on an Applied Biosystems 377 automated sequencer. The derived sequences were aligned with MegAlign (DNASTAR, Inc., Madison, WI) by using the clustal algorithm. Aligned sequences were transferred to PAUP (Sinauer Associates Inc., Sutherland, MA) for phylogentic analysis. Accession numbers D88295 (B. afzelii), X75201 (B. anserina), D82857 (B. bissettii), Y15097 (B. burgdorferi), D63372 (B. garinii), AF228034 (B. hermsii), D43777 (B. miyamotoi), D82863 (B. parkeri), U26705 (B. lonestari, New Jersey isolate), and U26704 (B. lonestari, Texas isolate) were used in this comparative genetic analysis.
Two hundred four A. americanum (21 adults and 183 nymphs) were collected: 13 adults and 44 nymphs from the properties of controls, and 8 adults and 139 nymphs near homes of persons meeting the STARI case definition. Twenty-nine A. maculatum adults were collected from control and STARI case areas. All but five ticks yielded PCR-quality DNA, as determined by PCR amplification of tick mitochondrial DNA (11).
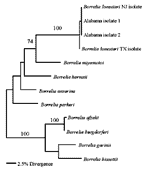
Two (11%) of 19 of adult A. americanum ticks analyzed were positive for B. lonestari flagellin gene DNA (Table). Positive results for B. lonestari were confirmed by amplification of the 16s rRNA gene as described by Barbour et al. (2). All 183 nymphs and all 26 adult A. maculatum were PCR negative for B. lonestari flagellin gene DNA. Moreover, all tick DNA samples were PCR negative when analyzed for the B. burgdorferi OspA gene. Sequence analyses for both positive samples showed >99% homology with the published B. lonestari sequences from New Jersey and Texas. Alabama isolates numbers 1 and 2 were 100% homologous to B. lonestari NJ and differed by 1 bp when compared with published sequences of the Texas isolate of B. lonestari. Phylogenetic analysis, using maximum likelihood and bootstrap analysis with 500 replications of derived sequences (Figure), illustrated that both isolates clustered with reported sequences of the B. lonestari NJ and TX strains and demonstrated considerable divergence from B. burgdorferi sensu stricto, the only genospecies shown to cause disease in the United States.
The nucleotide sequences of the B. lonestari flagellin gene have been submitted to GenBank and assigned accession numbers AF298653 (Alabama isolate 1) and AF298654 (Alabama isolate 2).
Spirochetes have been reported in A. americanum ticks from New York, New Jersey, Virginia, North Carolina, Alabama, Missouri, and Texas. In contrast to B. burgdorferi spirochetes, attempts to propagate these spirochetes from A. americanum in Barbour-Stoenner-Kelly (BSK-H) culture have been unsuccessful. Likewise, attempts to coculture A. americanum samples with an I. scapularis cell line (IDE2) failed to propogate B. lonestari (B. Johnson, pers. comm.). Thus, we used genetic analysis to determine the presence of this organism.
Erythema migrans associated with the bites of A. americanum has been reported from the southeastern United States, including Missouri (4) and North Carolina (5). Serum samples from these patients did not recognize B. burgdorferi antigens (4,5), and spirochetes from these cases have not been successfully cultured in BSK-H medium. Hence, these cases have been diagnosed as STARI. Recently, novel DNA sequences, amplified by PCR with primer sets recognizing the flagellin and 16s rRNA genes, have identified a new spirochete in A. americanum and B. lonestari (6). Nearly identical sequences have been amplified from A. americanum collected from Texas and New Jersey (2) and Maryland (1). B. lonestari is the suspected pathogen responsible for the STARI-related erythema migrans associated with bites by A. americanum (1). Moreover, B. lonestari was isolated from a patient in Westchester County, New York, who had traveled to Maryland and North Carolina and had an attached A. americanum at the site of an erythema migrans rash. This isolate differed only slightly from B. lonestari isolates reported in New Jersey and Texas (A. James et al., unpub. data).
Although the numbers of ticks we analyzed were small, our results suggest that adult A. americanum may transmit B. lonestari to persons in this area. Finding B. lonestari sequences in A. americanum from Alabama suggests that this spirochete is widely distributed in the United States. These first sequences from the southeastern United States are noteworthy because this region is a focus for numerous reports of erythema migrans associated with the bites of A. americanum. Further investigation is needed to formally isolate and propagate B. lonestari, as well as to determine its host reservoir.
Dr. Burkot is a senior research entomologist in the bacterial Zoonoses Branch, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention. His research interests include vector ecology, epidemiology, and the control of vector-borne bacterial, viral, and protozoal diseases.
Acknowledgment
The authors thank Katherine Hensleigh for initially recognizing and reporting these cases and the members of the epidemiologic investigation teams from the Alabama Department of Public Health (Jennifer Foster, William Johnson, Barbara Shoemaker, and Rhonda Stephens) and from the Centers for Disease Control and Prevention, Fort Collins, Colorado (David Dennis, Virginia Hinrichsen, and Sarah Lathrop), for identifying case-patient sites and for their invaluable assistance in these field studies.
References
- Felz MW, Durden LA. Attachment sites of four tick species (Acari:Ixodidae) parasitizing humans in Georgia and South Carolina. J Med Entomol. 1999;36:361–4.PubMedGoogle Scholar
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25:102–13.PubMedGoogle Scholar
- Oliver JH Jr, Clark KL, Chandler FW, Tao L, James AM, Banks CW, Isolation, cultivation and characterization of Borrelia burgdorferi from rodents and ticks in the Charleston area of South Carolina. J Clin Microbiol. 2000;38:120–4.PubMedGoogle Scholar
- Campbell GL, Paul WS, Schriefer ME, Craven RB, Robbins KE, Dennis DT. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990-1993. J Infect Dis. 1995;172:470–80. DOIPubMedGoogle Scholar
- Kirkland KB, Klimbo TB, Meriwether RA, Schriefer M, Levin M, Levine J, Erythema migrans-like rash illness at a camp in North Carolina: a new tick-borne disease? Arch Intern Med. 1997;157:2635–41. DOIPubMedGoogle Scholar
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivatable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–9. DOIPubMedGoogle Scholar
- Armstrong PM, Rich SM, Smith RD, Hart DL, Spielman A, Telford SR III. A new Borrelia infecting lone star ticks. Lancet. 1996;347:67–8. DOIPubMedGoogle Scholar
- Luckhart SG, Mullen GR, Wright JC. Etiologic agent of Lyme disease, Borrelia burgdorferi, detected in ticks (Acari:Ixodidae) collected at a focus in Alabama. J Med Entomol. 1991;28:652–7.PubMedGoogle Scholar
- Luckhart SG, Mullen GR, Durden LA, Wright JC. Borrelia sp. in ticks recovered from white-tailed deer in Alabama. J Wildl Dis. 1992;28:449–52.PubMedGoogle Scholar
- Zeidner NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in northern Colorado. J Infect Dis. 2000;182:616–9. DOIPubMedGoogle Scholar
- Black WC, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 7, Number 3—June 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nordin Zeidner, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, PO Box 2087, Rampart Road, Foothills Campus, Fort Collins, CO 80522-2087; fax: 970-221-6476
Top