Volume 7, Number 5—October 2001
Synopsis
Changing Epidemiology of Q Fever in Germany, 1947-1999
Abstract
The epidemiology of Q fever in Germany was examined by reviewing relevant studies since 1947 and by analyzing available surveillance data since 1962. The average annual Q fever incidence nationwide from 1979 to 1989 was 0.8 per million and from 1990 to 1999, 1.4 per million. The mean annual incidence from 1979 to 1999 ranged from a minimum of 0.1 per million in several northern states to 3.1 per million in Baden-Württemberg, in the South. We identified 40 documented outbreaks since 1947; in 24 of these sheep were implicated as the source of transmission. The seasonality of community outbreaks has shifted from predominantly winter-spring to spring-summer, possibly because of changes in sheep husbandry. The location of recent outbreaks suggests that urbanization of rural areas may be contributing to the increase in Q fever. Prevention efforts should focus on reducing sheep-related exposures, particularly near urban areas.
Q fever is caused by the pleomorphic, obligate intracellular rickettsial agent Coxiella burnetii, which has an envelope similar to that of gram-negative bacteria. It is found worldwide except in New Zealand (1). Its most important reservoirs are ticks and ruminant animals such as cows, sheep, and goats (1-4). Although infection rarely causes major clinical symptoms in animals (5), it has been associated with infertility (6,7) and abortion (8,9), particularly in first-bearing newly infected parturient animals (10). Birth products from infected animals thus contain high concentrations of C. burnetii and can be an important source of environmental contamination (11). Transmission to humans and other animals by the aerosol route is facilitated by the tenacity of C. burnetii's survival for months to years in a sporelike state on wool or fur contaminated with infected tick feces, in water, and in soil (12).
Acute Q fever in humans is characteristically a febrile, flulike illness associated with pneumonia or hepatitis (2,3,13-18). Rare complications include myocarditis, pericarditis, or meningoencephalitis (1,13,19). The death rate among persons with Q fever pneumonia is 0.5% to 1.5% (14). Up to half of patients may suffer protracted fatigue and weakness after acute disease (20,21). Rarely, more serious forms of chronic disease (most commonly endocarditis but also chronic hepatitis and vascular, osteoarticular, or pulmonary infections) may develop months to years after the acute infection (13,22,23). An increased long-term risk for arterial disease and death has also been observed (24).
Q fever was first recognized in southern Germany when several large outbreaks occurred in rural communities in 1947 to 1948 (25-28). Since then, it has been endemic in Germany. In the 1990s, several large outbreaks were recognized and investigated (5,29-36). This led us to review the epidemiology of this disease in Germany.
Clinically manifest Q fever has been statutorily notifiable since 1962 in the former West Germany and since 1979 in the former German Democratic Republic (GDR, East Germany). In 1991, the two reporting systems were amalgamated. The Robert Koch Institute in Berlin receives weekly reports of the number of persons with diagnosed (laboratory-confirmed or epidemiologically linked) Q fever from local health departments through the state health authorities. Local health departments receive notification of Q fever infections from hospitals and physicians in private practice. The Federal Office of Statistics prepares annual statistical reports of notifiable diseases. We reviewed surveillance data on Q fever from humans in Germany since 1962 from these sources.
The mean annual incidence rate was calculated for the periods from 1979 to 1989 and from 1990 to 1999 by taking the mean number of persons with Q fever reported per year during each period and dividing by the 1985 and 1995 German populations, respectively. The mean annual incidence rate for the period 1979 to 1999 was calculated by taking the mean number of persons with Q fever reported per year and dividing by the 1990 German population. Incidence rates for each of the 16 German states were calculated similarly.
We reviewed veterinary surveillance data based on passive, biannual reports of the number of domestic animal herds with laboratory-confirmed Q fever (37). To obtain further information on the epidemiology of Q fever in Germany, we searched databases (MEDLINE, from 1966; EMBASE, from 1974; AGROKAT, from 1960; CAB ANIMAL, from 1972; and CABVET SCI, from 1972) and reviewed the cited literature in the retrieved relevant articles. In addition, we contacted local health departments for unpublished details on recent outbreaks.
Surveillance
Human Q fever surveillance data since 1962 indicate an irregular cyclic incidence pattern (Figure 1). Very few cases were reported from states constituting the former GDR (approximately one fourth of the German population), with the exception of the years 1982 and 1983, when an outbreak occurred in Thuringia (38). To consider the long-term incidence pattern between 1962 and 1999, data from West German states must be considered separately. Except for 1979 to 1991, when there is little cyclicity, the interval between the peaks is approximately 4 to 6 years. The peak in 1993 with 181 (278, if unreported cases from two outbreaks [30,35] are taken into account) and the peak in 1999 with 268 cases (West German states) were the highest since 1964, when 437 cases were reported.
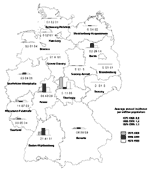
The average annual incidence of Q fever in Germany from 1979 to 1999 was 1.1 per million population. From 1979 to 1989, the incidence was 0.8 per million population, and from 1990 to 1999, 1.4 per million population. The mean annual incidence rates calculated from 1979 to 1999 were generally higher in the southern German states--highest in Baden-Württemberg (4.1 per million), followed by Hesse (2.8 per million), Rhineland-Palatinate (0.9 per million), and Bavaria (0.8 per million) (Figure 2). The high average incidence observed in West Berlin (1.4 per million) is explained by an outbreak in 1992 (5,39). Had the outbreak not occurred, an incidence rate of 0.2 per million would have been observed in Berlin in this period.
Outbreak
We documented 40 outbreaks of Q fever in humans from 1947 to 1999 (Table 1). In all but three outbreaks, the disease was confirmed in at least some of the persons affected, either serologically or by transmission from human serum or sputum to mice or guinea pigs.
Sheep were implicated in the transmission of Q fever to humans in at least 24 of the documented outbreaks (in six rural community outbreaks the source was uncertain). Exposure to products of conception was explicitly considered the potential source of infection in 11 of the sheep-associated outbreaks. In the outbreak in Dortmund, Northrhine-Westphalia, a case-control study revealed that exposure to manure contaminated with the products of conception from infected sheep was associated with Q fever (35). Infectious dust produced by shearing of sheep whose wool was presumably contaminated with infected tick feces was suspected as another possible source of transmission since shearing and disease occurrence were temporally associated in 3 of these 11 outbreaks and as the main source in one other outbreak. In 12 outbreaks, sheep located near or migrating through inhabited areas were implicated without specification of the presumed mechanism of transmission. Dry weather or wind blowing from areas where sheep were located to inhabited areas was thought to play a contributory role in at least 14 outbreaks.
Cattle were suspected as the source of infection in six outbreaks; four were community outbreaks (40-43). In the Niederrhein outbreak in 1958 (42,43), exposure to products of conception aborted by a seropositive cow and to other seropositive cows sold at an animal fair were considered to be the source of infection (42,43). Exposure to infected cows, some shedding C. burnetii in their milk, was implicated in the community outbreaks in Neulussheim, Zuzenhausen, and Schwaikheim in Baden-Württemberg (40,41). The other two cattle-associated outbreaks occurred in abattoirs (44,45). In one additional abbatoir-related outbreak, the source of infection could not be identified (46).
Two laboratory outbreaks occurred in 1947 and 1948 (26,47), but none have been reported since. Exposure to sputum of a patient who contracted Q fever in a laboratory that contained high concentrations of C. burnetii caused a hospital outbreak in 1948 (28).
The seasonality of community outbreaks in Germany has changed during the past decade compared to earlier years (Figure 3), with a marked decrease in winter outbreaks and an increase in summer ones. In contrast to earlier community outbreaks, which were almost exclusively rural, recent outbreaks have frequently involved people living in or near urban areas (5,30,34,35,48).
Seroprevalence Studies
Seroprevalence studies of C. burnetii antibodies in the German population have not been performed recently. Of 1,611 serum samples collected from blood donors from all 16 German states from 1983 to 1986, 22% were positive for C. burnetii antibodies using a phase I/II immunoglobulin (Ig) G enzyme-linked immunosorbent assay (ELISA) (49). In this survey, 15% of the 205 specimens from Hesse were positive. None of 1,075 sera from blood donors from Hesse had been positive for C. burnetii using complement fixation (CF) (positive titer 1:10) in a 1977 survey (50). This suggests a possible increase in exposure to C. burnetii during this interval, although ELISA has a higher sensitivity than the CF test (1). State-specific surveillance data from Hesse also suggest an increase in disease activity during this interval: The annual number of reported human Q fever cases was 0.6 cases per year between 1962 and 1977 but averaged 5.3 cases per year from 1978 to 1986. In a seroprevalence study among German federal armed forces personnel from 1985 to 1987 (51), 22% of 1,651 blood donors had antibodies to C. burnetii using the same ELISA as used by Schmeer et al. (49).
Q Fever in Animals
The extent of disease in animals over time is difficult to quantify. State veterinarians are required to report biannually the number of herds in which one or more animals have laboratory-confirmed Q fever (37). The average annual number of reported herds with Q fever was 71 between 1971 and 1979, 328 between 1980 and 1989, and 303 between 1990 and 1998. However, this does not permit inferences about the extent of infection, as neither the number of herds nor the number of animals tested is available. Furthermore, testing is not representative because the disease is generally asymptomatic in animals. Most testing occurs routinely in dairy cattle whose milk is destined for human consumption without pasteurization or in cattle destined for slaughter; otherwise, testing occurs only sporadically, as when the number of abortions increases, infertility is noted, or an outbreak of human disease is thought to be related to an animal source. In 1998, a survey requesting information on the number and results of Q fever testing in state veterinary laboratories in 13 of the 16 German states was performed (52). This showed that 7.8% of 21,191 tested cattle, 1.3% of 1,346 tested sheep, and 2.5% of 278 tested goats had evidence of C. burnetii infection by various tests (52). Again, the reasons for testing are not available; thus, the results cannot be considered representative of Q fever in animals in general and give only a rough indication of the extent of disease.
Seroprevalence surveys in cattle reveal that C. burnetii infection has been endemic in cattle in southern Germany since the 1950s; the seroprevalence reported in various surveys is generally >5% (53-55). Baden-Württemberg may be an exception (7), as a very low cut-off titer was chosen there, making comparison with other studies difficult (Table 1). Although different tests were used over time and between locations, seroprevalence in cattle in southern Germany appears to have remained fairly constant since the early 1950s. However, in other parts of Germany that have data available over time (e.g., Hesse, Northrhine Westphalia, Lower Saxony), seroprevalence was very low in the 1950s and 1960s but increased to levels comparable to those in southern Germany in the 1980s and 1990s (Table 1). In herds that had problems with infertility or abortions, seroprevalence rates were as high as 75% (6,7).
Seroprevalence of C. burnetii antibodies in surveys of sheep has generally been lower than in cattle (Table 2). This is likely partially due to the longer persistence of antibody titers in cattle (13). However, when surveys were performed in flocks of sheep implicated in human outbreaks, much higher seroprevalences were often found (Table 2), presumably reflecting more recent, acute infection with C. burnetii.
The number of persons reported annually with Q fever in Germany rose markedly in the 1990s. Although this could be due to enhanced awareness and better clinical recognition of the disease, the increased number of Q fever outbreaks, which are less likely to be clinically missed than sporadic cases, suggests that the increase is real. In fact, outbreaks in Baden-Württemberg, Hesse, Northrhine-Westphalia, and Berlin are responsible for most of the increase. The increased outbreak activity observed during the 1990s is reminiscent of the large outbreaks described between 1948 and 1954 (see Table A1 of documented Q fever outbreaks in Germany from 1948 through 1999).
The seroprevalence of 22% found in blood donors and military personnel in the 1980s in Germany is probably not representative of the general population. We have no information on the proportion of rural versus urban residence for blood donors. However, a seroprevalence study of blood donors in Switzerland (using a phase II IgG immunofluorescence test with a cut-off titer of 1:20) revealed lower seroprevalences in urban areas (10.9%) as compared to rural areas (17%) (68). In addition, because soldiers often train on grounds that are grazed upon by sheep (69), their exposure to C. burnetii may be higher than the general population's. A seroprevalence of 4% was found in 942 blood donors in Marseille, France, in 1988 also using a phase II IgG immunofluorescence test (cut-off titer 1:25) (70). In the Netherlands, a much higher seroprevalence of 45.5% was found in sera collected from blood donors between 1968 and 1983, using a Phase II IgG immunofluorescence test (cut-off titer 1:16) (71). However, international comparisons are difficult because of varying test methods and cutoffs.
The relatively high seroprevalence of C. burnetii antibodies in Germans suggests that the true number of persons with Q fever exceeds the number reported. Because Q fever often presents as a flulike illness, it may not be correctly diagnosed unless there is heightened suspicion of Q fever, such as would occur during an outbreak. Even then, not all cases may be reported. In 1993, at least 94 persons with symptomatic Q fever during recognized outbreaks were not reported to the surveillance system (Figure 1).
The cyclic incidence peaks seen on the surveillance curve generally correspond to years in which one or more outbreaks were documented. Thus, the occurrence of outbreaks was largely responsible for the cyclic pattern, a finding similar to that observed in Israel (72). It is unknown whether the incidence of sporadic Q fever follows the same cyclic pattern as Q fever occurring in outbreaks.
The highest Q fever incidences in Germany were observed in Bavaria, Baden-Württemberg, Rhineland-Palatinate, Hesse, Northrhine-Westphalia, and Thuringia, with Hesse showing the greatest increase since 1990. Of the 34 community outbreaks, 33 occurred in these six states. The tick Dermacentor marginatus, which acts as a host for C. burnetii and feeds on sheep in large numbers, is endemic in the four most southern states (Bavaria, Baden-Württemberg, Rhineland-Palatinate, and Hesse) but has not been found in the more northern states of Northrhine-Westphalia or Thuringia (59,73-75). Excreta from infected ticks persist in animal fur as a highly infectious dust, permitting aerosol transmission within the flock as well as to humans (73). This can occur through direct or indirect contact or through shearing, as observed in the outbreaks in Baden-Württemberg in 1998 and 1999 (see online table). C. burnetii has also been isolated from the tick Ixodes ricinus in Baden (40), in Northrhine-Westphalia (61), in Hesse (69), and in Thuringia (38). A role for I. ricinus in the transmission cycle of C. burnetii in areas where D. marginatus is not endemic but Q fever incidence is relatively high, such as Northrhine-Westphalia and Thuringia, is plausible, although evidence is lacking thus far. A tick-independent cycle of C. burnetii has been observed in cattle (55) and may thus exist in sheep in areas where D. marginatus is not endemic.
In addition to infectious dust from tick excreta, contaminated birth products are an important source of human infection (2,12,13,76). Terhaag (77) first noted the temporal association of Q fever outbreaks with outdoor lambing in southern Germany. Exposure to infectious products of conception was implicated in 10 sheep-associated community outbreaks and one cattle-associated community outbreak in this study.
Cattle were implicated in only four community and three abattoir outbreaks, none of which occurred recently. Although the increase in seroprevalence in cattle in more northern parts of Germany has been accompanied by an increase in reported Q fever outbreaks in Hesse and Northrhine-Westphalia (but not Lower Saxony or Schleswig-Holstein), cattle were never implicated as a possible source of the outbreaks. This is likely because cattle do not migrate over large distances, they graze on pastures close to inhabited areas less frequently, and they are not shorn. Moreover, calving normally occurs indoors under more controlled conditions.
From 1947 to 1969, epidemic Q fever activity occurred mainly during winter and spring (Figure 3). From 1970 to 1989, outbreaks occurred less commonly during winter, and started to occur during summer and fall as well. Finally, since 1990, outbreak activity has decreased further during winter and increased during summer (Figure 3). The change in seasonality coincides with changes in sheep husbandry. The nomadic form of sheep husbandry practiced mainly in southern parts of Germany has become less common. In former West Germany, nomadic herds gradually decreased from 1,178 in 1968 (30% of sheep) to 917 in 1988 (27% of sheep) (78). By 1994 this had decreased further to 702 (18% of sheep, reunified Germany) (79). Limited data from the late 1940s reveal that 50% of sheep farming was nomadic in Bavaria, 55% in Baden, and 88% in Württemberg (80). In 1994, this had decreased to 28% in Bavaria and 56% in (now unified) Baden-Württemberg (79). Because winter lambing (80-82), with either spring (80,81) or winter (82) shearing, is practiced in this form of sheep husbandry, the decrease in nomadic sheep farming in Germany may be related to the decrease in outbreaks during winter.
Spring lambing and shearing are most common in other forms of sheep farming, which have increased proportionately in Germany (82). Lambing at other times of the year, however, is possible and is being increasingly encouraged to enable year-round provision of the market with fresh lamb meat (83). Increased lambing or shearing during the warmer, drier seasons could increase the risk of aerogenous spread of C. burnetii from birth products or wool to humans and may thus be one possible explanation for the recent seasonal shift as well as for the increase in Q fever outbreaks in the 1990s.
In addition, outbreaks have increasingly affected people living in or close to urban areas (30,34,35,48). This suggests that increased exposure of susceptible humans to sheep through urbanization into traditionally rural areas may be another factor contributing to the observed increase in outbreaks. The use of sheep for landscaping purposes on recreational and park lands has also increased in importance since the 1970s (79,84).
Taken together, the presented data suggest that conditions for the transmission of C. burnetii to humans mainly from sheep have become more favorable in recent years, leading to increased outbreak activity in Germany. Therefore, stricter implementation of preventive measures is essential.
Apart from attempting to prevent the disease in animals as far as possible (e.g., isolation of animals that abort, examination of aborted lambs [69,85]), preventive measures must aim at preventing human contact with potentially infectious dust in animal fur and with infectious products of conception. In areas endemic for D. marginatus, control of transmission from ticks to animals must be achieved by rigorous treatment of sheep with acaricides (86-88). Since 1981, acaracide treatment of sheep has been recommended in areas where D. marginatus is endemic (86). However, such treatment may not protect for the entire tick season (86), so avoiding close contact between tick-infested sheep and susceptible persons (particularly during shearing) remains important. Rigorous disinfection after lambing or calving, including adequate heat or chemical treatment of manure before its use as fertilizer as well as removal of birth products through a licensed institution (89-91), is essential to prevent transmission.
Finally, investigation of vaccination of sheep against C. burnetii as a preventive measure in Germany may be warranted, particularly in light of a recently developed chloroform-methanol residue vaccine with fewer side effects (13,92,93) and long-lasting antibody induction in sheep (94). Studies in cattle suggest that this could reduce both the degree and proportion of infectious placentas (7,54). Unfortunately, no Q fever vaccine is currently licensed for use in animals in Germany.
Human disease caused by C. burnetii appears to have increased during the past decade in Germany. Q fever is endemic in cattle throughout Germany and in sheep at least in southern, eastern, and western parts of the country. Sheep have most often been implicated in transmission of the disease to humans. Urbanization into rural areas; increased grazing of sheep on recreational and park lands, leading to increased opportunity for contact between susceptible persons and infected animals; and changes in sheep husbandry may have contributed to the observed increase in outbreaks. Thus, awareness of this disease as a threat to human health and compliance with preventive and control measures must be improved among farmers, veterinarians, and the public.
Dr. Hellenbrand is a research assistant at the Infectious Diseases Epidemiology Unit, with research interests in Q fever, disease surveillance, and vaccine-preventable diseases.
Acknowledgment
We thank Drs. Wolf, Dittmeier, Dreweck, Zöllner, and Pfaff for providing us with the results of Q fever outbreak investigations performed in 1999.
References
- Fournier P-E, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–34.PubMedGoogle Scholar
- Marrie TJ. Coxiella burnetii. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Vol 2. New York: Churchill Livingstone; 1995. p. 1727-35.
- Werth D, Schmeer N, Müller H-P, Karo M, Krauss H. Nachweis von Antikörpern gegen Chlamydia psittaci und Coxiella burnetii bei Hunden und Katzen: Vergleich zwischen Enzymimmuntest; Immunperoxidase-Technik, Komplementbindungsreaktion und Agargelpräzipitationstest. J Vet Med. 1987;B34:165–76. DOIGoogle Scholar
- Schneider T, Jahn H-U, Steinhoff D, Guschoreck H-M, Liesenfeld O, Mäter-Böhm H, Q-Fieber-Epidemie in Berlin. Dtsch Med Wochenschr. 1993;118:689–95. DOIPubMedGoogle Scholar
- Gouverneur K, Schmeer N, Krauss H. Zur Epidemiologie des Q-Fiebers in Hessen: Untersuchungen mit dem Enzymimmuntest (ELISA) und der Komplementbindungsreaktion (KBR). Berl Munch Tierarztl Wochenschr. 1984;97:437–41.PubMedGoogle Scholar
- Woernle H, Müller K. Q-Fieber beim Rind: Vorkommen, Bekämpfung mit Hilfe der Impfung und/oder antibiotischen Behandlung. Tierarztl Umsch. 1986;41:201–12.
- Plagemann O. Die häufigsten infektiösen Abortursachen beim Schaf in Nordbayern unter besonderer Berücksichtigung der Chlamydien-und Salmonelleninfektionen. Tierarztl Prax. 1989;17:145–8.PubMedGoogle Scholar
- Heil-Franke G, Plagemann O, Singer H. Virologische und bakteriologische Untersuchungsergebnisse von abortierten Rinderfeten aus Nordbayern. Tierarztl Umsch. 1993;48:16.
- Sanford SE, Josephson GKA, MacDonald A. Coxiella burnetii (Q fever) abortion storms in goat herds after attendance at an annual fair. Can Vet J. 1994;35:376–8.PubMedGoogle Scholar
- Welsh HH, Lennette EH, Abinanti FR, Winn JF. Air-borne transmission of Q fever: the role of parturition in the generation of infective aerosols. Ann N Y Acad Sci. 1957;70:528–40. DOIPubMedGoogle Scholar
- Dedié K, Bockemühl J, Kühn H, Volkmer K-J, Weinke T. Bakterielle Zoonosen bei Tier und Mensch. Epidemiologie, Pathologie, Klinik, Diagnostik und Bekämpfung. Stuttgart, Germany: Ferdinand Enke Verlag; 1993.
- Marrie TJ, Raoult D. Q fever--a review and issues for the next century. Int J Antimicrob Agents. 1997;8:145–61. DOIPubMedGoogle Scholar
- Dupuis G, Peter O, Pedroni D, Petite J. Clinical aspects observed during an epidemic of 415 cases of Q fever. Schweiz Med Wochenschr. 1985;115:814–8.PubMedGoogle Scholar
- Mege JL, Maurin M, Capo C, Raoult D. Coxiella burnetii: the "query" fever bacterium: a model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–17. DOIPubMedGoogle Scholar
- Sawyer LA, Fischbein DB, McDade JE. Q fever: current concepts. Rev Infect Dis. 1987;9:935–46. DOIPubMedGoogle Scholar
- Klug J, Maenicke P. Q-Fieber--eine meldepflichtige Erkrankung. Z Ärztl Fortbild. 1985;79:119–20.
- Levy P-Y, Carrieri P, Raoult D. Coxiella burnetii pericarditis: report of 15 cases and review. Clin Infect Dis. 1999;29:393–7. DOIPubMedGoogle Scholar
- Marmion BP, Shannon M, Maddocks I, Storm P, Pentilla A. Protracted debility and fatigue after acute Q-fever [letter]. Lancet. 1996;347:977–8. DOIPubMedGoogle Scholar
- Ayres JG, Smith EG, Flint N. Protracted fatigue and debility after acute Q fever [letter]. Lancet 1996;347:978-9.8598797
- Edlinger EA. Chronic Q fever. Zentralblatt fur Bakteriologie und Hygiene 1987;267 A:51-6.
- Lovey P-Y, Morabia A, Bleed D, Péter O, Dupuis JP. Long term vascular complications of Coxiella burnetii infection in Switzerland: cohort study. BMJ. 1999;319:284–6. DOIPubMedGoogle Scholar
- Heni E, Germer WDQ. (eensland)-Fieber in Deutschland. Dtsch Med Wochenschr. 1948;73:472–6. DOIPubMedGoogle Scholar
- Kikuth W, Bock M. Twenty-three cases of laboratory infection with Q fever. Med Klin. 1949;44:1056–60.
- Freygang F. Klinische, epidemiologische und serologische Beobachtungen bei Q-Fieber 1948/49 in Nord-Württemberg. Dtsch Med Wochenschr. 1949;48:1457–63. DOIGoogle Scholar
- Siegert R, Simrock U, Stroeder U. Über einen epidemischen Ausbruch von Q-Fieber in einem Krankenhaus. Z Parasitol. 1950;2:1–40.
- Gesundheitsamt Ebersberg . Bericht über die Q-Fieber-Erkrankungen im Landkreis Ebersberg, 1999. Ebersberg: Gesundheitsamt Ebersberg; 2000.
- Freiburg G. Landesgesundheitsamt Baden-Württemberg, Robert Koch-Institut. Ergebnisse einer Fall-Kontroll-Studie zum Q-Fieber in Freiburg 1998. Freiburg, Germany: Gesundheitsamt Freiburg; 1998.
- Giessen G. Bericht über einen Q-Fieber-Ausbruch auf einer Lehr- und Forschungsstation der Universität Giessen. Giessen, Germany: Gesundheitsamt Giessen; 1997.
- Landratsamt Miltenberg--Gesundheitsamt. Q-Fieber-Kleinepidemie im Landkreis Miltenberg. Miltenberg, Germany: Landratsamt Miltenberg-Gesundheitsamt; 2000.
- Lyytikäinen O, Ziese T, Schwartländer B, Matzdorff P, Kuhnhen C, Jäger C, An outbreak of sheep-associated Q fever in a rural community in Germany. Eur J Epidemiol. 1998;14:193–9. DOIPubMedGoogle Scholar
- Kröner B. Q-Fieber--auch in Großstädten eine Gefahr. Dtsch Arztebl. 1995;92:378–81.
- Reintjes R, Hellenbrand W, Düsterhaus A. Q-Fieber-Ausbruch in Dortmund im Sommer 1999. Gesundh-Wes. 2000;62:1–6.
- Robert Koch-Institut. Q-Fieber-Epidemien in den Jahren 1992 und 1993. Epidemiologisches Bulletin. 1994;1:4–5.
- Federal Ministry of Nutrition Agriculture and Forestry. Q fever surveillance in domestic animals. Bonn: Federal Ministry of Nutrition, Agriculture and Forestry; 1999.
- Kramer M. Epizootologisch--epidemiologische Untersuchungsprogramme von potentiellen Naturherdinfektionen am Beispiel des Q-Fiebers im Bezirk Suhl [dissertation]. Leipzig, Germany: Karl-Marx-University; 1990.
- Mölle G, Hentschke J, Laiblin C. Diagnostische Maßnahmen anläßlich einer Q-Fieber-Endemie in einem berliner Schafbestand. J Vet Med. 1995;B42:405–13. DOIGoogle Scholar
- Hengel R, Kausche GA, Sheris E. Über zwei dörfliche Q-Fieberepidemien in Baden. Dtsch Med Wochenschr. 1950;45:1505–7. DOIGoogle Scholar
- Caesar O. Eine ausgedehnte Q-Fieber-Epidemie im Frühjahr 1950 in Nordwürttemberg. Medizinische Monatsschrift (Stuttgart). 1950;4:837–45.
- Trüb PGC, Boese W, Posch J. Die Q-Fieber-Epidemie am Niederrhein 1958, Land Nordrhein-Westfalen. Arch Hyg Bakteriol. 1960;144:48–73.PubMedGoogle Scholar
- Boese W, Trüb CLP, Posch J. Ergebnisse der serologischen Untersuchungen bei der Q-Fieber-Epidemie 1958 am linken Niederrhein, Land Nordrhein-Westfalen. Zentralbl Bakteriol. 1960;179:325–35.PubMedGoogle Scholar
- Mayer H. Beobachtungen zu einer Q-Fieber-Schlachthausepidemie in Württemberg. Dtsch Med Wochenschr. 1949;48:1476–7.
- Weise H-J. Epidemiologie des Q-Fiebers beim Menschen in der Bundesrepublik Deutschland. Bundesgesundheitsbl. 1971;14:71–5.
- Liebermeister K. Schlachthofepidemien durch Q-Fieber. Monatsheft fur Tierheilkunde. 1950;2:237–42.
- Nauck EG, Weyer F. Laboratoriumsinfektionen bei Q-Fieber. Dtsch Med Wochenschr. 1949;74:198–202. DOIPubMedGoogle Scholar
- Schulze K, Schwalen A, Klein RM, Thomas L, Leschke M, Strauer BE. Eine Q-Fieber-Pneumonie-Epidemie in Düsseldorf. Pneumonologie. 1996;50:469–73.
- Schmeer N, Krauss H, Werth D, Schiefer HG. Serodiagnosis of Q fever by enzyme-linked immunosorbent assay (ELISA). Zentralblatt fur Bakteriologie Hyg. 1987;267:57–63.PubMedGoogle Scholar
- Schmatz H-D, Schmatz S, Krauss H, Weber A, Brunner H. Seroepidemiologische Untersuchungen zum Vorkommen von Antikörpern gegen Rickettsien beim Menschen in der Bundesrepublik Deutschland. Immun Infekt. 1977;4:163–6.
- Werth D, Lampadius E, Krauss H. Seroprävalenz von Antikörpern gegen Coxiella burnetii bei Angehörigen der Bundeswehr (Blutspender und Krankenhauspatienten). Wehrmed Monatsschr. 1991;8:369–71.
- Hartung M. Bericht über die epidemiologische Situation der Zoonosen in Deutschland für 1998. Übersicht über die Meldungen der Bundesländer. Berlin: Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin; 1999.
- Germer WD, Glokner B. Epidemiologie des Q-Fiebers in Hessen: Untersuchungen mit dem Enzymimmuntest (ELISA) und der Komplementbindungsreaktion (KBR). Berl Munch Tierarztl Wochenschr. 1953;97:437–41.
- Roth CD, Bauer K. Untersuchungen zur Verbreitung des Q-Fiebers bei Rindern in Nordbayern und zu Maßnahmen zur Bekämpfung unter besonderer Berücksichtigung der Impfung. Tierarztl Umsch. 1986;41:197–201.
- Rehácek J, Krauss H, Kocianová E, Kovacova E, Hinterberger G, Hanák P, Studies of the prevalence of Coxiella burnetii, the agent of Q fever, in the foothills of the southern Bavarian Forest, Germany. Zentralbl Bakteriol. 1993;278:132–8. DOIPubMedGoogle Scholar
- Wegener KH. Das Q-Fieber und seine milchhygienische Bedeutung. Kiel Milchw Forschungsber. 1957;9:509–35.
- Neumann W. Serologische Untersuchungen über das Vorkommen von Q-Fieber-Antikörpern bei Rindern im Weser-Ems-Gebiet. Tierarztl Umsch. 1971;26:384–7.
- Wittenbrink MM, Gefaller S, Failing K, Bisping W. Einfluss von Bestands- und Tierfaktoren auf den Nachweis komplementbindender Antikörper gegen Coxiella burnetii beim Rind. Berl Munch Tierarztl Wochenschr. 1994;107:185–91.PubMedGoogle Scholar
- Kramer M. Zum Vorkommen, zur Verbreitung und zur Epidemiologie des Q-Fiebers in den neuen Ländern der Bundesrepublik Deutschland. Tierarztl Umsch. 1991;46:411–6.
- Schoop G. Das Q-Fieber. Übersicht über den Stand der Forschung und Untersuchungen über das Vorkommen in Südhessen. Monatsheft für Tierheilkunde. 1953;5:93–111.
- Schaaf J. Query-Fieber des Rindes. Monatsheft für Tierheilkunde. 1961;13:1–18.
- Schaal EH, Schäfer J. Zur Verbreitung des Q-Fiebers in einheimischen Rinderbeständen. Dtsch Tierarztl Wochenschr. 1984;91:52–6.PubMedGoogle Scholar
- Klemt C, Krauss H. Zur Epidemiologie des Q-Fiebers: Vorkommen von Antikörpern gegen Coxiella burnetii beim Rind im Regierungsbezirk Arnsberg, Nordrhein/Westfalen (1989/90). Tierarztl Umsch. 1991;46:520–4.
- Schliesser T, Schmidt U. Zur Verbreitung und Bedeutung des Q-Fiebers bei Schaf und Rind. Zentralbl Veterinarmed [C]. 1970;17:238–42. DOIGoogle Scholar
- Schmeer N, Krauss H, Wilske B. Untersuchungen zur Serodiagnose des Q-Fiebers beim Menschen - Nachweis von nicht-komplementbindenden IgM-Antikörpern im Enzyme-linked Immunosorbent Assay (ELISA). Immun Infekt. 1984;12:245–51.PubMedGoogle Scholar
- Lange S, Klaus G. Seroepidemiologische Untersuchungen zum Nachweis von Q-Fieber bei Schafen in Mittel-Thüringen. Berl Munch Tierarztl Wochenschr. 1992;105:333–5.PubMedGoogle Scholar
- Schaal E, Goetz W. Über Q-Fieber-Infektionen und deren Ursache unter der Bevölkerung des Raumes Simmerath/Eifel aus tierärztlicher Sicht. Dtsch Tierarztl Wochenschr. 1974;81:477–500.PubMedGoogle Scholar
- Dupuis G, Péter O, Mottiez M-C, Vouilloz M. Séro-prévalence de la fièvre Q humaine en Suisse. Schweiz Med Wochenschr. 1986;116:494–8.PubMedGoogle Scholar
- Thoms H-J. Epidemiologische Untersuchungen zum Vorkommen von Coxiella burnetii auf vier Truppenübungsplätzen der Bundeswehr in Nordrhein-Westfalen und Niedersachsen [dissertation]. Giessen, Germany: Justus-Liebig-Universität Giessen; 1996.
- Tissot-Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller P-J, Epidemiologic features and clinical presentations of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–34. DOIPubMedGoogle Scholar
- Richardus JH, Donkers A, Dumas AM, Schaap GJ, Akkerman JP, Huisman J, Q fever in the Netherlands: a sero-epidemiological survey among human population groups from 1968 to 1983. Epidemiol Infect. 1987;98:211–9. DOIPubMedGoogle Scholar
- Yarrow A, Slater PE, Costin C. Q fever in Israel. Public Health Rev. 1990-91;18:129–37.PubMedGoogle Scholar
- Liebisch A, Rahman MS. Zum Vorkommen und zur vektoriellen Bedeutung der Zecken Dermacentor marginatus (Sulzer, 1776) und Dermacentor reticulatus (Fabricius, 1794) in Deutschland. Tropenmed Parasitol. 1976;27:393–404.PubMedGoogle Scholar
- Liebisch A, Burgdorfer W, Rahman MS. Epidemiologische Untersuchungen an Schafzecken (Dermacentor marginatus) auf Infektionen mit Rickettsien. Dtsch Tierarztl Wochenschr. 1978;85:121–6.PubMedGoogle Scholar
- Liebisch A. Die Rolle einheimischer Zecken (Ixodidae) in der Epidemiologie des Q-Fiebers in Deutschland. Dtsch Tierarztl Wochenschr. 1983;83:274–6.
- Doerr HW, Hoferer E, Leschhorn V, Nassal J. Q-Fieber: Eine in Süddeutschland endemische Infektionskrankheit. Dtsch Med Wochenschr. 1981;106:1532–5. DOIPubMedGoogle Scholar
- Terhaag L. Zur Epidemiologie des Q-Fiebers. Q-Fieber in der Eifel. Arch Hyg Bakteriol. 1953;137:247–69.PubMedGoogle Scholar
- von Korn S. Schafe in Koppel- und Hütehaltung. Stuttgart, Germany: Eugen Ulmer; 1992.
- Landesschafzuchtverbände VD. Schafe: Fakten, Zahlen und agrarpolitische Entscheidungen zur Schafhaltung in Deutschland. Bonn: Vereinigung Deutscher Landesschafzuchtverbände; 1995.
- Diener HO. Süddeutsche Schäferfibel. Munich: Buchdruckerei J. Gotteswinter; 1949.
- Doehner H. Haltung, Fütterung und Krankheiten des Schafes. Berlin: Paul Parey; 1944.
- Behrens H, Schellje R, Waßmuth R. Lehrbuch der Schafzucht. Berlin: Paul Parey; 1983.
- Schlolaut W. Nutzungsziel Lammfleischerzeugung, 2. Konsequenzen für Management und Zucht. Deutsche Schafzucht. 1993;85:56–9.
- Hesse Department of Nutrition Agriculture and Land Development. Schafzucht und Schäfer in Hessen. Wiesbaden, Germany: Hesse Department of Nutrition Agriculture and Land Development; 1992.
- Kaulfuss K. Infectious abortions of sheep. Man is endangered. Deutsche Schafzucht. 1997;89:406–9.
- Liebisch A. Control of Dermacentor marginatus in sheep in Germany. In: Whitehead GB, Gibson JD, editors. Proceedings of the International Conference on Tick Biology and Control under the Auspices of the Tick Research Unit, Rhodes University, Grahamstown, South Africa. Grahamstown, South Africa: Rhodes University; 1981. p. 157-8.
- Liebisch A. Das Q-Fieber als Naturherdinfektion in Süddeutschland. Bundesgesundheitsbl. 1977;20:185–91.
- Liebisch A. Ecology and distribution of Q-fever rickettsiae in Europe with special reference to Germany. Recent Advances in Acarology. 1979;II:225–31.
- Böhm R, Strauch D. Desinfektion im Stall--weniger Krankheiten, mehr Leistung. Bonn: Gesellschaft fur Druckabwicklung mbH; 1996.
- Kazár J, Brezina R. Control of rickettsial diseases. Eur J Epidemiol. 1991;7:282–6. DOIPubMedGoogle Scholar
- Fries LF, Waag DM, Williams JC. Safety and immunogenicity in human volunteers of a chloroform-methanol residue vaccine for Q fever. Infect Immun. 1993;61:1251–8.PubMedGoogle Scholar
- Waag DM, England MJ, Pitt MLM. Comparative efficacy of a Coxiella burnetii chloroform: methanol residue (CMR) vaccine and a licensed cellular vaccine (Q-Vax) in rodents challenged by aerosol. Vaccine. 1997;15:1779–83. DOIPubMedGoogle Scholar
- Lang GH, Prescott JF, Williams JC. Serological response in sheep vaccinated against Coxiella burnetii (Q fever). Can Vet J. 1994;35:373–4.PubMedGoogle Scholar
- Landesgesundheitsamt Baden-Württemberg. Jahresbericht 1999 des Landesgesundheitsamts Baden-Württemberg. Stuttgart, Germany: Landesgesundheitsamt Baden-Württemberg; 2000.
- Gesundheitsamt Ebersberg . Bericht über die Q-Fieber-Erkrankungen im Landkreis Ebersberg, 1999. Ebersberg: Gesundheitsamt Ebersberg; 2000.
- Landratsamt Miltenberg - Gesundheitsamt. Q-Fieber-Kleinepidemie im Landkreis Miltenberg. Miltenberg: Landratsamt Miltenberg - Gesundheitsamt; 2000.
- Reintjes R, Hellenbrand W, Düsterhaus A. Q-Fieber-Ausbruch in Dortmund im Sommer 1999. Gesundheitswesen. 2000;62:1–6. DOIPubMedGoogle Scholar
- Freiburg G. Landesgesundheitsamt Baden-Württemberg, Robert Koch-Institut. Ergebnisse einer Fall-Kontroll-Studie zum Q-Fieber in Freiburg 1998. Freiberg, Germany: Gesundheitsamt Freiburg; 1998.
- Giessen G. Bericht über einen Q-Fieber-Ausbruch auf einer Lehr- und Forschungsstation der Universität Giessen. Giessen, Germany: Gesundheitsamt Giessen; 1997.
- Centers for Disease Control and Prevention. Q fever outbreak-- Germany, 1996. MMWR. 1997;46:29–33.PubMedGoogle Scholar
- Lyytikäinen O, Ziese T, Schwartländer B, Matzdorff P, Kuhnhen C, Jäger C, An outbreak of sheep-associated Q fever in a rural community in Germany. Eur J Epidemiol. 1998;14:193–9. DOIPubMedGoogle Scholar
- Schulze K, Schwalen A, Klein RM, Thomas L, Leschke M, Strauer BE. Eine Q-Fieber-Pneumonie-Epidemie in Düsseldorf. Pneumonologie. 1996;50:469–73.
- Robert Koch-Institut. Q-Fieber-Epidemien in den Jahren 1992 und 1993. Epidemiologisches Bulletin. 1994;1:4–5.
- Thode C, Zöller L. Q-Fieber: Eine wehrmedizinisch relevante Zoonose. Wehrmed Monatsschr. 2000;44:35–8.
- Thoms H-J. Epidemiologische Untersuchungen zum Vorkommen von Coxiella burnetii auf vier Truppenübungsplätzen der Bundeswehr in Nordrhein-Westfalen und Niedersachsen [dissertation]. Giessen, Germany: Justus-Liebig-Universität Giessen; 1996.
- Kröner B. Q-Fieber - auch in Großstädten eine Gefahr. Dtsch Arztebl. 1995;92:378–81.
- Schneider T, Jahn H-U, Steinhoff D, Guschoreck H-M, Liesenfeld O, Mäter-Böhm H, Q-Fieber-Epidemie in Berlin. Dtsch Med Wochenschr. 1993;118:689–95. DOIPubMedGoogle Scholar
- Mölle G, Hentschke J, Laiblin C. Diagnostische Massnahmen anlässlich einer Q-Fieber-Endemie in einem berliner Schafbestand. J Vet Med. 1995;B42:405–13. DOIGoogle Scholar
- Engelhart S, Wiebel M, Schulz V. Q-Fieberendemie in der Vorderpfalz. Pneumonologie. 1992;46:153–7.
- Kramer M. Epizootologisch - epidemiologische Untersuchungsprogramme von potentiellen Naturherdinfektionen am Beispiel des Q-Fiebers im Bezirk Suhl [dissertation]. Leipzig, Germany: Karl-Marx-University; 1990.
- Doerr HW, Hoferer E, Leschhorn V, Mayer H, Theuer D. Epidemiologische und klinische Erfahrungen anläßlich einer Q-Fieber Epidemie im Herbst 1978 in Nordbaden. Bundesgesundheitsbl. 1980;23:57–64.
- Nassal J. Q-Fieber: Eine klassische Zooanthroponose. Tierarztl Umsch. 1982;37:109–14.
- Kathke N. Q-Fieber-Infektionen in einer Großstadt. Öffentliches Gesundheitswesen 1978;40:286-8.
- Schaal E, Goetz W. Über Q-Fieber-Infektionen und deren Ursache unter der Bevölkerung des Raumes Simmerath/Eifel aus tierärztlicher Sicht. Dtsch Tierarztl Wochenschr. 1974;81:477–500.PubMedGoogle Scholar
- Doerr HW, Amelung E, Schmitz H, Haas R. Q-Fieber-Endemie in Südbaden. Dtsch Med Wochenschr. 1974;99:556–8. DOIPubMedGoogle Scholar
- Weise H-J. Epidemiologie des Q-Fiebers beim Menschen in der Bundesrepublik Deutschland. Bundesgesundheitsbl. 1971;14:71–5.
- Schliesser T. Das Q-Fieber - Erfahrungen bei einer Epidemie in München. Landarzt. 1968;44:1198–202.PubMedGoogle Scholar
- Trüb PGC, Boese W, Posch J. Die Q-Fieber-Epidemie am Niederrhein 1958, Land Nordrhein-Westfalen. Arch Hyg Bakteriol. 1960;144:48–73.PubMedGoogle Scholar
- Boese W, Trüb CLP, Posch J. Ergebnisse der serologischen Untersuchungen bei der Q-Fieber-Epidemie 1958 am linken Niederrhein, Land Nordrhein-Westfalen. Zentralbl Bakteriol. 1960;179:325–35.PubMedGoogle Scholar
- Schmid W. Über eine Q-Fieberepidemie im Landkreis Aschaffenburg. Medizinische Monatsschrift (Stuttgart). 1955;9:225–8.
- Nolden J. Eine Q-Fieber-Epidemie in der Nordwesteifel. Dtsch Med Wochenschr. 1954;79:1743–5. DOIPubMedGoogle Scholar
- Terhaag L. Zur Epidemiologie des Q-Fiebers. Q-Fieber in der Eifel. Arch Hyg Bakteriol. 1953;137:247–69.PubMedGoogle Scholar
- Caesar O. Eine ausgedehnte Q-Fieber-Epidemie im Frühjahr 1950 in Nordwürttemberg. Medizinische Monatsschrift (Stuttgart). 1950;4:837–45.
- Hengel R, Kausche GA, Sheris E. Über zwei dörfliche Q-Fieberepidemien in Baden. Dtsch Med Wochenschr. 1950;45:1505–7. DOIGoogle Scholar
- Liebermeister K. Schlachthofepidemien durch Q-Fieber. Monatsheft für Tierheilkunde. 1950;2:237–42.
- Mayer H. Beobachtungen zu einer Q-Fieber-Schlachthausepidemie in Württemberg. Dtsch Med Wochenschr. 1949;48:1476–7.
- Freygang F. Klinische, epidemiologische und serologische Beobachtungen bei Q-Fieber 1948/49 in Nord-Württemberg. Dtsch Med Wochenschr. 1949;48:1457–63. DOIGoogle Scholar
- Siegert R, Simrock U, Stroeder U. Über einen epidemischen Ausbruch von Q-Fieber in einem Krankenhaus. Z Parasitol. 1950;2:1–40.
- Heni E, Germer WDQ. (eensland)-Fieber in Deutschland. Dtsch Med Wochenschr. 1948;73:472–6. DOIPubMedGoogle Scholar
- Kikuth W, Bock M. Twenty-three cases of laboratory infection with Q fever. Med Klin. 1949;44:1056–60.
- Nauck EG, Weyer F. Laboratoriumsinfektionen bei Q-Fieber. Dtsch Med Wochenschr. 1949;74:198–202. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 5—October 2001
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Wiebke Hellenbrand, Department of Infectious Diseases Epidemiology, Robert Koch-Institut, Stresemann Str. 90-102, 10963 Berlin, Germany; fax: 30-4547-3533
Top