Pneumocystis jirovecii Pneumonia in Patients with or without AIDS, France
Volume 20, Number 9—September 2014
Antoine Roux, Emmanuel Canet, Sandrine Valade, Florence Gangneux-Robert, Samia Hamane, Ariane Lafabrie, Daniéle Maubon, Anne Debourgogne, Soléne Le Gal, Fréderic Dalle, Marion Leterrier, Dominique Toubas, Christelle Pomares, Anne Pauline Bellanger, Julie Bonhomme, Antoine Berry, Isabelle Durand-Joly, Denis Magne, Denis Pons, Christophe Hennequin, Eric Maury, Patricia Roux
1, and Élie Azoulay

Author affiliations: Centre Medico-Chirurgical FOCH, Suresnes, France (A. Roux); Hôpital Saint-Louis, Paris, France (E. Canet, S. Valade, S. Hamane, A. Lafabrie, É. Azoulay); Centre Hospitalier Universitaire (CHU) de Rennes, Rennes, France (F. Gangneux-Robert ); CHU de Grenoble, Grenoble, France (D. Maubon); CHU de Nancy, Nancy, France (A. Debourgogne); Centre Hospitalier Régional et Universitaire de Brest, Brest, France (S. Le Gal); CHU de Dijon, Dijon, France (F. Dalle); CHU de Nantes, Nantes, France (M. Leterrier); CHU de Reims, Reims, France (D. Toubas); Hôpital de l’Archet, Nice, France (C. Pomares); CHU Jean Minjoz, Besançon, France (A.P. Bellanger); CHU Côte de Nacre, Caen, France (J. Bonhomme); CHU de Toulouse, Toulouse, France (A. Berry); CHU de Lille, Lille, France (I. Durand-Joly); Hôpital Saint-Antoine, Paris (D. Magne, C. Hennequin, P. Roux, E. Maury); CHU Gabriel Montpied, Clermont-Ferrand, France (D. Pons)
Suggested citation for this article
Medscape CME Activity
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: August 14, 2014; Expiration date: August 14, 2015
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe presentations of Pneumocystis jirovecii pneumonia, based on a prospective, multicenter observational study in France
• Assess outcomes of Pneumocystis jirovecii pneumonia
• Identify risk factors for mortality in Pneumocystis jirovecii pneumonia.
CME Editor
Shannon O’Connor, ELS, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Shannon O’Connor has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Antoine Roux, MD, PhD; Emmanuel Canet, MD; Sandrine Valade, MD; Florence Gangneux-Robert, PharmD, PhD; Samia Hamane, MD; Ariane Lafabrie, MS; Daniele Maubon, MD, PhD; Anne Debourgogne, PhD, MPH; Solène Le Gal, DVM, PhD; Frederic Dalle, PharmD, PhD; Marion Leterrier, PharmD; Dominique Toubas, MD, PhD; Christelle Pomares, MD, PhD; Anne Pauline Bellanger, PharmD, PhD; Julie Bonhomme, MD, PhD; Xavier Iriart, PhD; Isabelle Durand-Joly, PhD; Denis Magne, PhD; Denis Pons, Pharmacien Biologiste; and Eric Maury, MD, PhD, have disclosed no relevant financial relationships. Christophe Hennequin, MD, PhD, has disclosed the following relevant financial relationships: served as an advisor or consultant for Gilead Sciences, Inc., Merck Sharp & Dohme Corp; served as a speaker or a member of a speakers bureau for Pfizer Inc, Merck Sharp & Dohme Corp; received grants for clinical research from Pfizer Inc. Elie Azoulay, MD, PhD, has disclosed the following relevant financial relationships: served as a speaker or a member of a speakers bureau for Gilead Sciences, Inc., Merck Sharp & Dohme Corp; received grants for clinical research from Pfizer Inc.
Abstract
Pneumocystis jirovecii pneumonia (PCP) in patients without AIDS is increasingly common. We conducted a prospective cohort study of consecutive patients with proven PCP; of 544 patients, 223 (41%) had AIDS (AIDS patients) and 321 (59%) had other immunosuppressive disorders (non-AIDS patients). Fewer AIDS than non-AIDS patients required intensive care or ventilation, and the rate of hospital deaths—17.4% overall—was significantly lower for AIDS versus non-AIDS patients (4% vs. 27%; p<0.0001). Multivariable analysis showed the odds of hospital death increased with older age, receipt of allogeneic bone marrow transplant, immediate use of oxygen, need for mechanical ventilation, and longer time to treatment; HIV-positive status or receipt of a solid organ transplant decreased odds for death. PCP is more often fatal in non-AIDS patients, but time to diagnosis affects survival and is longer for non-AIDS patients. Clinicians must maintain a high index of suspicion for PCP in immunocompromised patients who do not have AIDS.
Pneumocystis jirovecii pneumonia (PCP), caused by the fungus P. jirovecii (formerly P. carinii), is a life-threatening, opportunistic infection that is often the AIDS-defining illness in patients with HIV infection. Consequently, PCP has been extensively studied as a manifestation of the AIDS epidemic. However, in high-resource countries, the decrease in the prevalence of AIDS and the use of highly active antiretroviral therapy and routine primary PCP prophylaxis have diminished the number of patients with HIV-related PCP (1,2). At the same time, PCP has emerged as a concern in patients with non–HIV-related immune deficiencies. PCP has become more common among these patients as a result of treatment changes such as increasing use of immunosuppressive agents to treat malignancies, autoimmune diseases, and inflammatory diseases, as well as an increase in the number of solid organ transplants (SOTs) (3,4). Thus, patients who have hematologic or solid-organ malignancies or autoimmune or chronic inflammatory diseases, or those who have received an SOT or hematopoietic stem cell transplant (HSCT), are at high risk for PCP (5–8).
Whether these changes in PCP epidemiology have affected the clinical manifestation and outcome of the disease remains unclear. As early as 1989, a biological study that compared PCP in patients with and without AIDS found significant differences in fungi counts and lung inflammation (9). Another study evaluated clinical manifestations and outcomes of PCP in patients without AIDS seen during 1980–1993 (10) but did not include a comparison with AIDS patients. In a study comparing PCP in AIDS and non-AIDS patients in Basel, Switzerland, during 1982–1998, non-AIDS patients more often required intensive care and mechanical ventilation, although rates of death were not significantly different for the 2 groups (11). The Switzerland study and another comparison of AIDS and non-AIDS patients with PCP published in 1984 (12) found that symptom duration was longer and oxygen tension needs higher for AIDS patients.
These studies suggest that differences in PCP pathogenesis and influences of the underlying disease or treatment may affect the expression of PCP. However, recent data are lacking on the differences between clinical features and outcomes of PCP in AIDS and non-AIDS patients. Clinicians need this information to help identify patients who require prophylaxis and to enable early diagnosis of PCP at a stage when treatment is most likely to be effective. To obtain data on manifestations and outcomes of PCP in recent years and to identify risk factors for death, we performed a prospective, multicenter, observational study of consecutive patients with confirmed PCP admitted to 17 hospitals in France during 2007–2010.
Patients and Management
The appropriate ethics committee approved this study; informed consent was not required because of the observational design. For the study, the head mycologist at each of 17 university-affiliated hospitals in France prospectively included consecutive patients with confirmed PCP who were admitted during January 1, 2007–December 31, 2010. We defined confirmed PCP as a positive result for Pneumocystis jirovecii by Gomori-Grocott or toluidine blue stain or positive immunofluorescence test results (4) for a bronchoalveolar lavage (BAL) fluid or induced sputum specimen. Induced sputum testing or bronchoscopy with BAL was performed at the discretion of the clinicians by using previously described procedures (13,14). We did not include patients for whom only PCR results were positive.
For all included patients, chest radiographs were obtained; computed tomography (CT) scans were performed when deemed necessary by clinicians. Bilateral interstitial or alveolointerstitial opacities on chest radiographs and diffuse ground-glass opacities on CT images were considered typical findings for PCP. Septal lines and centrolobular nodules were also interpreted to support a diagnosis of PCP. Focal consolidation, pleural effusion, subpleural nodules, and cavitation were considered atypical findings. Criteria for microbiologically documented pneumonia were as follows: clinical symptoms of pneumonia; pulmonary infiltrates; and >1 positive noncolonizing microbiological sample (i.e., blood culture, tracheal aspirate, sputa examination, BAL, protected sample, or pleural fluid). Urine antigens (Streptococcus pneumoniae and Legionella pneumophila) were included in routine testing for the microbiological documentation of pneumonia. When the microbiological data were negative but the patient had symptoms of pneumonia and pulmonary infiltrates, the case was classified as clinically documented pneumonia (13).
Data Collection
Data were collected prospectively. Steroid treatment was either high dose (>1 mg/kg for >1 mo) or long term (>3 mo at any dose) (3,14). Mycologists and study investigators obtained missing and follow-up data by reviewing patients’ medical charts and by interviewing the specialists who provided usual care to the patients. Bacterial, viral, fungal, and parasitic infections were diagnosed on the basis of criteria reported elsewhere (13). Information on PCP prophylaxis was recorded; trimethoprim-sulfamethoxazole (TMP-SMX), aerosolized pentamidine (1×/mo), dapsone, and atovaquone were classified as effective prophylaxis options (3,4). Information on treatments used for PCP and the time from admission to treatment initiation were recorded; TMP-SMX, pentamidine, atovaquone, dapsone, and clindamycin-primaquine were considered acceptable options for PCP treatment (4). Shock was defined as persistent hypotension despite appropriate fluid load, requiring treatment with a vasopressive drug.
Steroids as adjunctive therapy were used on the basis of standard protocol recommendations for patients with AIDS at a dose that depended on patient location (e.g., medical unit). In deeply hypoxemic, critically ill patients, steroids were implemented at the dosage of 240 mg/day for 3 days, then at 1 mg/kg/day for 7 days, followed by tapering doses to be stopped before day 21 (15,16). In patients who were less critically ill, the dose was 1 mg/kg/day followed by a tapering dose after day 7 to be stopped before day 21. Similar protocols were used for AIDS and non-AIDS patients.
Statistical Analyses
The variables in the dataset are described or summarized by using either median and interquartile range or number and proportion of the total (%). Categorical variables were compared by using the Fisher exact test and continuous variables by using the nonparametric Wilcoxon test or Mann-Whitney test for pairwise comparisons. All tests were 2-sided, and p<0.05 was considered statistically significant. Kaplan-Meier curves and log-rank tests were used to compare hospital death rates between groups of patients. Logistic regression analysis was used to identify variables significantly associated with hospital deaths by estimating the odds ratios (OR) with 95% CIs. Variables yielding p values <0.20 in the univariable analyses were entered into a multivariable logistic regression model with stepwise variable selection using an automatic procedure based on the Akaike Information Criterion, with hospital deaths as the variable of interest. The co-variates were entered into the model with a p value cutoff for removal of 0.1. Co-linearity and interactions were tested. For the multivariable analysis, missing data were handled by using multiple imputation with chained equations. The imputed missing data focused on 3 variables: oxygen saturation on admission (35 patients), time to treatment (17 patients), and time from respiratory symptom onset to diagnosis (11 patients). The Hosmer-Lemeshow test was used to check goodness-of-fit of the logistic regression model.
Causes of Immunodeficiency
We included 544 patients in the study. Median age was 51 (interquartile range 40–62) years, and 370 (68%) were men A total of 223 (41%) patients had AIDS and 321 (59%) had other immunosuppressive conditions (Figure 1). Among patients with AIDS, PCP was the first manifestation of AIDS for 105 (44.8%); only 4 (5.0%) had CD4 lymphocyte counts >200 cells/mm3. As shown in Table 1, the main causes of immunodeficiency in the non-AIDS patients were SOT (n = 99, 30.8%), chiefly of a kidney (80/99); and hematologic malignancies (n = 84, 26.2%), chiefly lymphoproliferative diseases (72/84). Twenty-seven (8.4%) patients were HSCT recipients (14 allogeneic, 13 autologous); 65 (20.2%) had autoimmune or chronic inflammatory disease; and 46 (14.3%) had solid-organ malignancies.
At the time of PCP diagnosis, high-dose or long-term steroid therapy was the most common immunosuppressive treatment for non-AIDS patients: 88% (n = 63) for those with SOTs, 85% (n = 71) for those with hematologic malignancies, 100% (n = 13) for those with HSCTs, 91% (n= 41) for those with solid-organ malignancies, and 89% (n = 52) for those with autoimmune or chronic inflammatory disease. SOT recipients were also receiving anticalcineurin agents (n = 59, 82%), purine inhibitors (n = 56, 77%), rituximab (n = 1, 6%), mechanistic target of rapamycin inhibitors (n = 6, 8%), tumor necrosis factor–α antagonists (n = 3, 4%), or intravenous immunoglobulins (n = 1, 1%). Patients with hematological malignancies also had received rituximab (n = 29, 35%) and fludarabin (n = 8, 9.5%). All HSCT patients were receiving anticalcineurin agents. Patients with autoimmune or chronic inflammatory diseases were receiving methotrexate (n = 14, 21.5%), rituximab (n = 6, 9.2%), tumor necrosis factor–α antagonists (n = 6, 9.2%), anticalcineurin agents (n = 5, 7.7%), or purine inhibitors (n = 4, 6.1%). (Note that values are approximate; data are missing among each subpopulation.)
Clinical Manifestations of PCP
The clinical manifestations of PCP in AIDS and non-AIDS patients are shown in Table 2 and Figure 1. Overall, 96% of patients were not receiving PCP prophylaxis. The median time from the onset of respiratory symptoms to PCP diagnosis was significantly shorter for non-AIDS patients (5 [range 1–15] days) than for AIDS patients (21 [7–30] days) (p<0.0001). However, as shown in Tables 1 and 2, hypoxemia was more severe in non-AIDS patients, who required higher oxygen flow rates (4 [range 3–5] L/min) than did AIDS patients (2 [1.3–3] L/min). Non-AIDS patients also more often required intensive care and noninvasive or invasive ventilation. Shock was more common in non-AIDS patients and was significantly associated with pulmonary microbial co-infection (OR 3.09 [95% CI 1.44–6.68]; p = 0.004), in agreement with the presence of microbiologically documented pneumonia (13) in half of the patients with shock. CT scans were obtained for 279 patients; typical findings were seen in 37 patients, of whom 29 were believed to have pulmonary bacterial microbial co-infection and 8 microbial co-infections with a second opportunistic microorganism.
Diagnosis and Treatment of PCP
A BAL sample was diagnostic for 87% and 97% of AIDS and non-AIDS patients, respectively (p = 0.0003). Overall, microbial co-infection as previously defined (13) was suspected or confirmed for 169 (31%) patients, including 92 (16.9%) with bacterial infection, 65 (6.6%) with viral infection, and 38 (6.9%) with fungal infection. For 32 (5.8%) patients, >2 microbial co-infections were identified; the most frequent site of microbial co-infection was the lung (Technical Appendix Table). Univariable analysis showed an association between microbial co-infection and death (OR 2.29, 95% CI 1.41–3.71) (Technical Appendix Table), but multivariable analysis did not confirm this finding (OR 1.99, 95% CI 0.91–4.33; p = 0.09).
Time from hospital admission to the start of treatment for PCP was longer for non-AIDS patients than for AIDS patients (2 [range 0–6]) days vs. 1 [0–2] days; p<0.0001). TMP-SMX was the first-line anti-PCP agent used for 97% of patients. However, patients with AIDS more often required a switch to second-line therapy than did non-AIDS patients (25.5% vs. 7%; p<0.0001); this change was most often made because of allergic reactions or hepatic or renal toxicity. Adjunctive steroid therapy was used in 40.4% of patients overall and in a significantly higher proportion of AIDS than in non-AIDS patients (55% vs. 43%; p = 0.01).
Risk Factors for Death
Hospital death data were available for 478 (88%) patients, and the percentage of hospital deaths was significantly higher for non-AIDS than AIDS patients (27% vs. 4%; p<0.0001). For non-AIDS patients, death rates varied by cause of immunodeficiency, from a low of 3.75% for kidney transplant recipients to a high of 43% for allogeneic HSCT recipients. Invasive mechanical ventilation was used in 28% and 43% of patients in these 2 groups, respectively (p<0.0001).
Of 7 variables independently associated with hospital death by multivariable analysis, 2 were associated with lower death rates: AIDS diagnosis (OR 0.33, 95% CI 0.12–0.92) and receipt of a SOT (OR 0.08, 95% CI 0.02–0.31) (Table 3). Five variables were associated with increased death rates: older age (OR 1.04/additional year, 95% CI 1.02–1.06); receipt of a HSCT (OR 8.6, 95% CI 1.40–53.02); need for oxygen on admission (OR 4.06, 95% CI 1.44–11.5); need for invasive mechanical ventilation (OR 16.70, 95% CI 7.25–38.47); and longer time from admission to initiation of PCP treatment (OR 1.11/additional day, 95% CI 1.04–1.18).
Improved cumulative survival was significantly associated with underlying condition (p<0.0001 for AIDS vs. non-AIDS comparison; Figure 2). Shorter time from admission to treatment initiation was also associated with improved cumulative survival (Figure 2).
This multicenter, prospective study describes the current picture of PCP in immunocompromised patients with or without AIDS in a high-resource country. In this cohort, AIDS-related PCP was less common than was PCP associated with other types of immunosuppression. Our findings confirm several differences between AIDS and non-AIDS patients in clinical presentation and outcomes related to PCP, as described by Kovacs et al. (12). The progression of PCP was faster for non-AIDS patients; these patients had a significantly shorter time from onset of symptoms to diagnosis but still experienced a faster progression of illness, including more severe hypoxemia, greater need for intensive care and invasive mechanical ventilation, higher prevalence of shock, and a longer time to PCP treatment initiation. Death rates were also significantly higher for non-AIDS patients, and one of the variables independently associated with death was longer time to PCP treatment initiation for non-AIDS patients.
This study offers several contributions toward the development of PCP prophylaxis guidelines for specific at-risk groups. One of the reasons for the lower proportion of AIDS patients than of non-AIDS patients in this study population could have been the widespread use of highly active antiretroviral therapy and PCP prophylaxis among AIDS patients (1,2). However, among patients with AIDS in our study, only 3 (2.7%) were receiving PCP prophylaxis; for 100 (44.8%) patients, the PCP diagnosis was the reason for the AIDS diagnosis.
In our study, PCP treatment was started later after admission in non-AIDS patients than in AIDS patients, and longer time to treatment independently predicted odds for death, which is in agreement with findings of an earlier study (12). Longer time to treatment was the only predictor of death in our study that could be mitigated. Treatment initiation differed by only 1 day for AIDS versus non-AIDS patients, yet this difference was associated with reduced death rates for AIDS patients. Therefore, because routine implementation of PCP treatment on admission may be associated with higher survival, clinicians should implement treatment as soon as the diagnosis is suspected, without waiting the 2 days required to confirm the diagnosis.
Our findings indicate that PCP prophylaxis could improve outcomes for high-risk patients without AIDS. Among non-AIDS patients in this study, 99 (30.8%) were SOT recipients, a population for which recent guidelines recommend PCP prophylaxis for 6–12 months, a period that might be extended on the basis of level of immunosuppression and immunosuppressive drug requirements (18,19). Despite this recommendation, however, recent studies suggest that 1 month of prophylaxis would be sufficient for kidney transplant patients (20). Given the high rate of death in our cohort, this conclusion should be challenged. Maintaining a high index of suspicion for PCP in immunocompromised patients appears to be of the utmost importance. In addition, as with AIDS patients, every effort should be made to ensure compliance with PCP prophylaxis in non-AIDS patients. Non-AIDS patients may be less aware than AIDS patients that they are at risk for PCP. This point may be of particular relevance, as the course of PCP was significantly more acute in non-AIDS patients, with a median symptom duration of 5 (range 1–15) days compared with 21 (7–30) days for AIDS patients (p<0.0001). Educating non-AIDS patients about PCP might result in earlier medical evaluation and hospital admission and, consequently, in shorter lengths of time to PCP diagnosis and treatment. In addition, development of rapid and minimally invasive diagnostic tests could improve the early diagnosis and treatment of PCP (21).
We found marked differences in death rates across patient groups. Deaths were lowest for AIDS patients and highest for HSCT recipients, and rates in our study were consistent with earlier data (6,12). Microbial co-infection rates in our study were also in agreement with earlier data (22). More than one fourth of our patients overall had microbial co-infection, which indicates a need for comprehensive diagnostic investigations in patients with PCP and for routine broad-spectrum antimicrobial drug therapy when findings are atypical for PCP.
Adjunctive steroid therapy has been proven to increase survival in AIDS patients with severe PCP (23,24), but 2 small retrospective studies found that adjunctive steroids had no effect on survival for non-AIDS patients (17,25). In our study, although non-AIDS patients had more severe hypoxemia and more often required invasive mechanical ventilation, they received adjunctive steroid therapy significantly less often than did AIDS patients.
Our study has several limitations. First, the patients were recruited at university hospitals, which may have influenced the distribution of risk factors for PCP. However, most of these risk factors are associated with diseases that require management in university hospitals. Our data obtained for consecutive patients from 17 centers are probably representative of PCP in other countries where optimal AIDS treatment and critical care are widely available. In addition, the diagnosis and therapeutic management of these patients was not standardized, and variations in testing and treatment strategies may have affected outcomes and determinants of death. Moreover, the diagnostic strategy may have been different for AIDS versus non-AIDS patients, as well as for critically ill patients versus non–critically ill patients; these differences could have resulted in different proportions of patients with documented microbial superinfections. However, our objective was to describe all PCP patients seen during the past few years, and we found no effect of the center at which a patient was treated on mortality rates (data not shown). This study also included only episodes of PCP for which a patient was hospitalized. AIDS patients with mild PCP episodes could be treated as outpatients, and the prognosis and characteristics for these patients may vary substantially from those of our study population. Last, we included only PCP cases proven by tinctorial or immunofluorescence staining. PCP may be present in some patients who have positive PCR test results for P. jirovecii but for whom stain results are negative or unavailable (26). However, because isolated PCR test positivity can indicate colonization and not infection (27), we confined our study to cases of confirmed infection to maximize the validity of our data.
In summary, PCP occurs in patients with a range of conditions associated with immunosuppression. For AIDS patients, efforts should focus on improving the early detection of HIV infection and adherence to PCP prophylaxis. For non-AIDS patients, guidelines regarding PCP risk evaluation and prophylaxis are needed. We found higher death rates and longer time from hospital admission to initiation of PCP treatment for non-AIDS patients. Clinicians must maintain a high index of suspicion for PCP in immunocompromised patients who do not have AIDS, and these patients should be educated about the early symptoms that can indicate PCP. Treatment should be implemented early in high-risk patients, even before appropriate diagnostic tests are completed.
Dr Roux is a pulmonary physician who is the fellow of Prof. Azoulay at Réanimation Médicale, Hôpital Saint-Louis, Paris. He specializes in pulmonary involvement in immunocompromised patients and in lung transplant patients.
Acknowledgments
We thank Rebecca Hamidfar-Roy, Anne Thiebaut-Bertrand, Patrick Germaud, Antoine Parrot, Gilles Nevez, Magali Chabe, Emilie Frealle, and Laurence Delhaes for contributing to the inclusion of patients in this study and for providing clinical reports for patients treated at their centers.
This research was supported by a grant from the French Ministry of Health.
References
Crothers K,
Huang L,
Goulet JL,
Goetz MB,
Brown ST,
Rodriguez-Barradas MC,
HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med.
2011;
183:
388–
95.
DOIPubMedGoogle Scholar Thompson MA,
Aberg JA,
Cahn P,
Montaner JS,
Rizzardini G,
Telenti A,
Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society–USA panel. JAMA.
2010;
304:
321–
33.
DOIPubMedGoogle Scholar Catherinot E,
Lanternier F,
Bougnoux ME,
Lecuit M,
Couderc LJ,
Lortholary O.
Pneumocystis jirovecii pneumonia. Infect Dis Clin North Am.
2010;
24:
107–
38.
DOIPubMedGoogle Scholar Bollée G,
Sarfati C,
Thiery G,
Bergeron A,
de Miranda S,
Menotti J,
Clinical picture of Pneumocystis jiroveci pneumonia in cancer patients. Chest.
2007;
132:
1305–
10.
DOIPubMedGoogle Scholar Roblot F,
Godet C,
Le Moal G,
Garo B,
Faouzi Souala M,
Dary M,
Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis.
2002;
21:
523–
31.
DOIPubMedGoogle Scholar Yale SH,
Limper AH.
Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc.
1996;
71:
5–
13.
DOIPubMedGoogle Scholar Zahar JR,
Robin M,
Azoulay E,
Fieux F,
Nitenberg G,
Schlemmer B.
Pneumocystis carinii pneumonia in critically ill patients with malignancy: a descriptive study. Clin Infect Dis.
2002;
35:
929–
34.
DOIPubMedGoogle Scholar Limper AH,
Offord KP,
Smith TF,
Martin WJ II.
Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis.
1989;
140:
1204–
9.
DOIPubMedGoogle Scholar Arend SM,
Kroon FP,
van’t Wout JW.
Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med.
1995;
155:
2436–
41.
DOIPubMedGoogle Scholar Nüesch R,
Bellini C,
Zimmerli W.
Pneumocystis carinii pneumonia in human immunodeficiency virus (HIV)–positive and HIV-negative immunocompromised patients. Clin Infect Dis.
1999;
29:
1519–
23.
DOIPubMedGoogle Scholar Kovacs JA,
Hiemenz JW,
Macher AM,
Stover D,
Murray HW,
Shelhamer J,
Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med.
1984;
100:
663–
71.
DOIPubMedGoogle Scholar Azoulay E,
Mokart D,
Lambert J,
Lemiale V,
Rabbat A,
Kouatchet A,
Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med.
2010;
182:
1038–
46.
DOIPubMedGoogle Scholar Green H,
Paul M,
Vidal L,
Leibovici L.
Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc.
2007;
82:
1052–
9.
DOIPubMedGoogle Scholar Ogawa J,
Harigai M,
Nagasaka K,
Nakamura T,
Miyasaka N.
Prediction of and prophylaxis against Pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod Rheumatol.
2005;
15:
91–
6.
DOIPubMedGoogle Scholar Tasaka S,
Tokuda H.
Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother.
2012;
18:
793–
806.
DOIPubMedGoogle Scholar Delclaux C,
Zahar JR,
Amraoui G,
Leleu G,
Lebargy F,
Brochard L,
Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin Infect Dis.
1999;
29:
670–
2.
DOIPubMedGoogle Scholar de Boer MG,
Kroon FP,
le Cessie S,
de Fijter JW,
van Dissel JT.
Risk factors for Pneumocystis jirovecii pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis.
2011;
13:
559–
69.
DOIPubMedGoogle Scholar Martin SI,
Fishman JA;
AST Infectious Disease Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant.
2013;
13(
Suppl 4):
272–
9.
DOIPubMedGoogle Scholar Anand S,
Samaniego M,
Kaul DR.
Pneumocystis jirovecii pneumonia is rare in renal transplant recipients receiving only one month of prophylaxis. Transpl Infect Dis.
2011;
13:
570–
4.
DOIPubMedGoogle Scholar To KK,
Wong SC,
Xu T,
Poon RW,
Mok KY,
Chan JF,
Use of nasopharyngeal aspirate for diagnosis of Pneumocystis pneumonia. J Clin Microbiol.
2013;
51:
1570–
4.
DOIPubMedGoogle Scholar Barbier F,
Coquet I,
Legriel S,
Pavie J,
Darmon M,
Mayaux J,
Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med.
2009;
35:
1678–
86.
DOIPubMedGoogle Scholar Bozzette SA,
Sattler FR,
Chiu J,
Wu AW,
Gluckstein D,
Kemper C,
A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med.
1990;
323:
1451–
7.
DOIPubMedGoogle Scholar Gagnon S,
Boota AM,
Fischl MA,
Baier H,
Kirksey OW,
La Voie L.
Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med.
1990;
323:
1444–
50.
DOIPubMedGoogle Scholar Pareja JG,
Garland R,
Koziel H.
Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest.
1998;
113:
1215–
24.
DOIPubMedGoogle Scholar Azoulay E,
Bergeron A,
Chevret S,
Bele N,
Schlemmer B,
Menotti J.
Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest.
2009;
135:
655–
61.
DOIPubMedGoogle Scholar Alanio A,
Desoubeaux G,
Sarfati C,
Hamane S,
Bergeron A,
Azoulay E,
Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect.
2011;
17:
1531–
7.
DOIPubMedGoogle Scholar
Suggested citation for this article: Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, Maubon D, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis [Internet]. 2014 Sep [date cited]. http://dx.doi.org/10.3201/eid2009.131668
10.3201/eid2009.131668
Medscape CME Quiz
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Pneumocystis jirovecii Pneumonia in Patients with or without AIDS, France
CME Questions
1. Your patient is a 42-year-old man with AIDS admitted to the hospital for respiratory distress and suspected Pneumocystis jirovecii pneumonia, also known as pneumocystis pneumonia (PCP). According to this prospective, multicenter observational study, which one of the following statements about presentations of PCP in patients with AIDS and with other immunosuppressive disorders is correct?
A. More than half of patients with PCP had AIDS
B. One third of patients with PCP had been taking PCP prophylaxis
C. Median time from the onset of respiratory tract symptoms to PCP diagnosis was 5 days (range, 1–15 days) in patients without AIDS and 21 days (range, 7–30 days) in patients with AIDS (p<0.0001)
D. Hypoxemia at presentation was more severe in patients with AIDS
2. According to this prospective, multicenter observational study, which one of the following statements about outcomes of PCP is correct?
A. Fewer patients without AIDS vs patients with AIDS required intensive care unit admission and ventilation
B. Hospital mortality rate was 17.4% overall, 4% in patients with AIDS, and 27% in patients without AIDS
C. Hospital mortality rates did not differ significantly between patients with AIDS and those without AIDS
D. Shock was more common in patients with AIDS and was significantly associated with pulmonary microbial coinfection
3. According to this prospective, multicenter observational study, which one of the following statements about risk factors for mortality in PCP, as determined by multivariable analysis, would most likely be correct?
A. Hospital mortality was not associated with age
B. Solid-organ transplantation was associated with twice the risk for hospital mortality
C. Hospital mortality was not associated with time to treatment
D. Patients receiving immediate oxygen had approximately 4-fold the risk for hospital mortality
Activity Evaluation
|
1. The activity supported the learning objectives.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
2. The material was organized clearly for learning to occur.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
3. The content learned from this activity will impact my practice.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
|
4. The activity was presented objectively and free of commercial bias.
|
|
Strongly Disagree
|
|
|
|
Strongly Agree
|
|
1
|
2
|
3
|
4
|
5
|
Figure 1

Figure 1. Flowchart of selection of patients with Pneumocystis jirovecii pneumonia (PCP) for study and underlying conditions among non-AIDS patients, France, January 1, 2007–December 31, 2010. Miscellaneous conditions: inflammatory diseases or automimmune (n = 4); common variable immunodeficiency (n = 2); focal segmental glomerulosclerosis (n = 2); sarcoidosis (n = 1); steroid-dependent asthma (n = 1); idiopathic pulmonary fibrosis (n = 1); acute alcoholic hepatitis (n = 3). ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; CLL, chronic lymphoid leukemia; CML, chronic myeloid leukemia; HSCT, hematopoietic stem cell transplant; SOT, solid organ transplant.
Figure 2
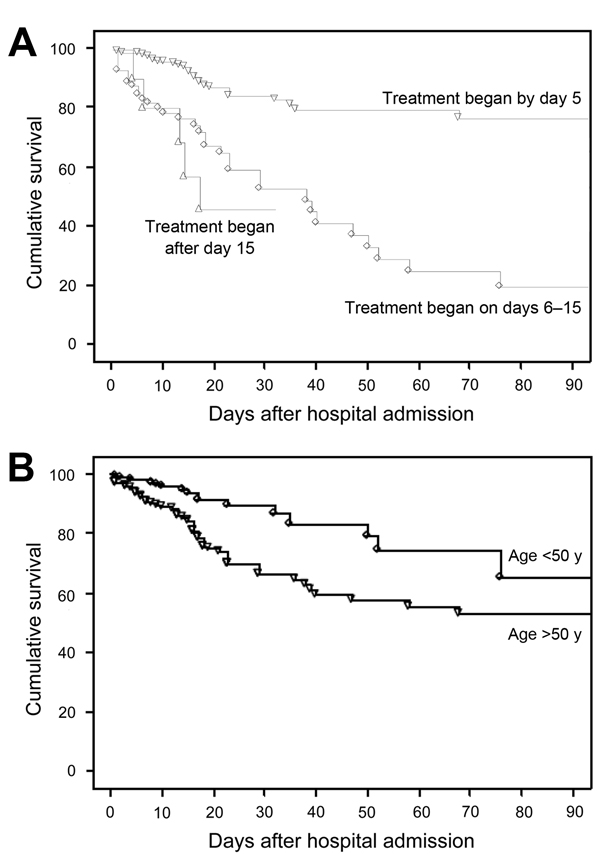
Figure 2. Survival in 544 patients with Pneumocystis jirovecii pneumonia by A) number of days from admission to treatment initiation and B) patient age, France, January 1, 2007–December 31, 2010. p<0.0001 by log-rank test for both comparisons.
Table 1
Clinical characteristics of 544 patients with and without AIDS at diagnosis with PCP, France, January 1, 2007–December 31, 2010*
| Characteristic |
AIDS patients, n = 223 |
Non-AIDS patients, n = 321 |
p value |
| Clinical features |
|
|
|
| Prophylaxis prescribed† |
3 (1) |
12 (4) |
0.06 |
| Temperature >38°C |
165 (74) |
263 (82) |
0.05 |
| Days from constitutional symptom onset to diagnosis, median (IQR) |
30 (14–60) |
7 (2–15) |
<0.0001 |
Shock
|
5 (2.2)
|
23 (7)
|
0.01
|
| Respiratory symptoms |
|
|
|
| Cough |
170 (76.2) |
173 (54) |
<0.0001 |
| Dyspnea |
176 (79) |
234 (73) |
0.10 |
Days from respiratory symptom onset to diagnosis, median (IQR)
|
21 (7–30)
|
5 (1–15)
|
<0.0001
|
| Laboratory test results |
|
|
|
| SpO2, median (IQR) |
95 (90–97) |
91 (86–96) |
0.003 |
| Lymphocyte count, cells/mm3, median (IQR) |
802 (499–1,200) |
500 (278–880) |
0.0004 |
| CD4+ T-cell count, cells/mm3, median (IQR) |
167 (89–342) |
32 (12–75) |
<0.0001 |
C-reactive protein
|
48 (17–128)
|
120 (59–210)
|
<0.0001
|
| Radiologic findings |
|
|
|
| Chest radiograph results typical for PCP |
183 (82) |
247 (77) |
0.23 |
| Chest radiograph results atypical for PCP‡ |
31 (14) |
48 (15) |
0.66 |
| Pneumothorax |
7 (3.1) |
7 (2.2) |
0.50 |
| Chest radiograph results unremarkable |
9 (4) |
26 (8) |
0.34 |
| Atypical computed tomography scan pattern§ |
15 (14) |
22 (14) |
0.47 |
Table 2
Clinical management of 544 AIDS and non-AIDS patients after diagnosis with PCP, France, January 1, 2007–December 31, 2010*
Characteristic
|
AIDS patients, n = 223
|
Non-AIDS patients, n = 321
|
p value
|
| Days from admission to treatment initiation, median (IQR) |
1 (0–2) |
2 (0–6) |
<0.0001 |
| Intensive care admission |
65 (35) |
134 (50) |
0.0015 |
| Immediate oxygen needed |
87 (49) |
160 (69) |
<0.0001 |
Oxygen flow rate, L/min, mean (95% CI)
|
2 (1.3–2.8)
|
3.8 (2.8–4.8)
|
0.015
|
| Mechanical ventilation |
|
|
|
| Noninvasive needed |
17 (8) |
50 (16) |
0.0053 |
| Noninvasive failed |
16 (8) |
46 (15) |
0.013 |
Invasive needed
|
25 (11.0)
|
98 (30.5)
|
<0.0001
|
| Hospital deaths |
8 (4) |
75 (27) |
<0.0001 |
|
Table 3
Multivariate analysis of independent predictors of hospital death for AIDS and non-AIDS patients with PCP, France, January 1, 2007–December 31, 2010*
| Variable |
Odds ratio (95% CI) |
| HIV infection |
0.33 (0.12–0.92) |
| Solid organ transplant |
0.08 (0.02–0.31) |
| Age, per additional year |
1.04 (1.02–1.06) |
| Allogeneic HSCT |
8.6 (1.40–53.02) |
| Need for immediate oxygen therapy |
4.06 (1.44–11.5) |
| Need for intubation and mechanical ventilation |
16.70 (7.25–38.47) |
| Time to PCP treatment, per additional day |
1.11 (1.04–1.18) |

