Volume 10, Number 12—December 2004
Research
Exposure to Nonhuman Primates in Rural Cameroon
Abstract
Exposure to nonhuman primates has led to the emergence of important diseases, including Ebola hemorrhagic fever, AIDS, and adult T-cell leukemia (HTLV). To determine the extent of exposure to nonhuman primates, persons were examined in 17 remote villages in Cameroon that represented three habitats (savanna, gallery forest, and lowland forest). Questionnaire data were collected to assess whether persons kept wild animal pets; hunted and butchered wild game; had experienced bites, scratches, or injuries from live animals; or had been injured during hunting or butchering. While all villages had substantial exposure to nonhuman primates, higher rates of exposure were seen in lowland forest sites. The study demonstrates that exposure is not limited to small groups of hunters. A high percentage of rural villagers report exposure to nonhuman primate blood and body fluids and risk acquiring infectious diseases.
Closely related species generally share susceptibility to the same groups of microorganisms (1). The anthropoid primates, which include humans, and to a lesser degree simian primates share broadly similar physiologic and genetic characteristics and thus susceptibility to many viruses, bacteria, fungi, protozoa, helminths and ectoparasites (2,3). Members of the family Hominidae, which includes humans, chimpanzees, bonobos, and gorillas, share an even greater similarity in susceptibility to microorganisms (3). Our closest relatives, chimpanzees and, most likely, bonobos, share with us the potential for infection with virtually the same set of microorganisms.
A range of activities involves direct contact between humans and nonhuman primates and allow for the transmission of microorganisms. Such behavior can facilitate transmission of microorganisms from nonhuman primates to humans (4), with consequences for human health, as well as from humans to nonhuman primates, with consequences for wildlife conservation (5). Care for captive nonhuman primates has lead to the transmission of a range of infections, including simian foamy virus (6), herpesvirus B (HBV) (7), primate malaria (8), and tuberculosis (9). Nonhuman primate ecotourism (e.g., gorilla watching) has been associated with the possible transmission from humans to nonhuman primates of diseases that include scabies (Sarcoptes scabiei) (10), intestinal parasites (11), and measles (12). Laboratory handling of tissues or fluids of nonhuman primates has lead to transmission of a range of infections to humans, including simian immunodeficiency virus (SIV) (13) and SV40, which was subsequently distributed through oral polio vaccine to millions of people (14). Additionally, keeping nonhuman primate pets has been linked to transmission of a variety of microorganisms (15). Finally, hunting and butchering nonhuman primates have been linked to the transmission of Ebola (16,17), monkeypox (18), and simian foamy virus (19). Because of the broad range of fluid and tissue types involved with hunting and butchering, this mechanism of transmission may be particularly important in cross-species transmission (1), although other behavior, such as wildlife necropsy, has similar risks (20).
A number of important human diseases, including AIDS (HIV), adult T-cell leukemia (HTLV-1) and malaria (Plasmodium spp.), are believed to have emerged as the results of ancient or contemporary cross-species transmission from nonhuman primates. While the emergence of malaria was presumably the result of vector-borne transmission, the mechanisms which led to the emergence of HIV and HTLV remain unknown. One of the current primary hypotheses to explain the origins of HIV is that hunting and butchering nonhuman primates led to cross-species transmission (21,22). This hypothesis is strengthened by recent evidence suggesting that hunted nonhuman primates have a high rate of SIV infection (23) and evidence of hunting-associated cross-species transmission of a nonhuman primate retrovirus, simian foamy virus, in central African hunters (19).
While some groups are at risk for contact with nonhuman primates, the frequency of behavior involving exposure to nonhuman primates remains largely unknown. The objective of the present study was to use behavioral tools to examine the frequency and extent of exposure to nonhuman primates among persons living in rural village sites in a region of high primate biologic diversity.
Participants
Seventeen village sites in Cameroon were selected for this study (Table 1, Figure 1). Sites were selected in highly rural areas, often at the end of small, unpaved roads. Sites were chosen to obtain different habitats, including 2 savannah sites, 2 gallery forest sites, and 13 lowland forest sites, and based on their proximity to regions supporting wild game populations. The sites selected are all in the southern part of Cameroon, a region that includes extensive lowland rainforest (Figure 1). The 17 sites include 2 in each of the Southwest, Northwest, West, Littoral, and Central Provinces, 3 in the South Province, and 4 in the East Province. All 17 sites in the study participated in commercial sales of hunted wild game.
Studies were conducted in the context of a community-based HIV prevention campaign designed to provide information and decrease transmission. Participation in the study was voluntary. Persons who participated in the HIV prevention campaign were asked if they would like to hear more about a research study, and the study was described to those who were interested. Study description, the informed consent procedure, and questionnaire administration were all done orally in English or French, which are widely spoken as second languages in the study villages. Participants were offered compensation approximately equivalent to 1 day of work, since participation often precluded farm work on that day. The study protocol was approved by the Johns Hopkins Committee for Human Research, the Cameroon National Ethical Review Board, and the HIV Tri-Services Secondary Review Board. In addition, a single project assurance was obtained from the Cameroonian Ministry of Health and accepted by the National Institutes of Health Office for Protection from Research Risks.
Behavioral Data
After the informed consent process, participants were asked to respond to a behavioral questionnaire. The questionnaire was administered individually by trained interviewers, without regard to the sex of the participant. The questionnaire, which was linked, was designed to provide basic demographic information as well as information on behavior either directly or indirectly associated with exposure to the blood or body fluids of nonhuman primates. The questionnaire was pretested in Cameroon before use. Behaviors considered indirectly associated with exposure to blood or body fluids include butchering, hunting, and keeping pets. Behavior considered to be directly associated with exposure to blood or body fluids includes having been scratched or bitten by a nonhuman primate or having been injured while hunting or butchering. Following pretests, locally appropriate taxonomic categories were derived from accepted local terms for animals. For example, “monkey” was identified as an appropriate taxonomic category, and participants were not asked to distinguish between monkey species. Other taxonomic categories used include chimpanzee, gorilla, and rodent. Rodents were included because they are perhaps the most commonly hunted and eaten type of forest animal and serve as a useful comparison with nonhuman primates. Participants were asked to identify which of these four taxa they had consumed, hunted, butchered, or kept as pets. Participants were asked to estimate their monthly frequency of consumption of each of the wild taxa and of the wild game overall. Participants were also asked to report on direct contact with wild taxa, including the taxa involved, having been scratched or bitten, or having been injured while hunting or butchering.
A total of 3,971 persons were interviewed. Both men and women participated and were represented approximately equally in the sites, with 46.3% female participants and 53.7% male participants. These aggregate results were similar to those found within the sites. Participants’ ages ranged from 16 to 97 years. Participants were not equally distributed with regards to age; younger age groups were more represented. Participants were classed into four age groups: 16–30 years, 31–45 years, 46–60 years, and >60 years. Ages 16–30 made up 42% of participants, ages 31–45 made up 27% of participants, ages 46–60 made up 21% of participants, and ages >60 made up 10% of participants.
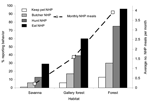
Participants reported having kept pets in all four taxonomic categories. Monkeys were kept as pets more frequently than other types of wild animals. The overall percentage of participants keeping pets from all sites combined was 0.6% keeping gorillas, 1.5% keeping chimpanzees, 9.9% keeping monkeys, and 1.8% keeping rodents. Sites in the study differed in their tendency to keep pets; persons in lowland sites reported keeping pets more frequently than people in gallery and savanna sites did (Figure 2).
In addition to keeping wild animal pets, three additional contact-associated activities were examined in this study, including hunting, butchering, and eating. Participants in all sites reported having hunted, butchered, and eaten animals from the four wild game taxa examined in this study. A higher percentage had eaten wild game than had butchered wild game, and a higher percentage had butchered wild game than hunted wild game (Figure 2). Hunting, butchering, and eating wild game were more common in forest sites than in other sites. For hunting, butchering, and eating, participants in the study had greater contact with rodents and monkeys than chimpanzees and gorillas (Figure 3).
While no significant departure was seen from expected proportions of women and men reporting eating of wild game of all types (χ² = 0.046, nonsignificant) or of rodents (χ² = 0.001, nonsignificant), fewer women than expected and more men than expected reported eating monkeys (χ² = 6.762, p < 0.01), chimpanzees (χ² = 102.216, p < 0.001), and gorillas (χ² = 0.046, p < 0.001). Of persons reporting hunting nonhuman primates, a higher proportion than expected by chance were men (53.7% expected, 98.7% observed) and lower proportion than expected were women (46.3% expected; 1.3% observed) (χ² = 1,119.130, p < 0.001). However, of participants reporting butchering nonhuman primates, a significantly higher proportion than expected were women (46.3% expected, 50.9% observed) and a lower proportion than expected were men (53.7% expected, 49.1% observed) (χ² = 61.376, p < 0.001). The average number of nonhuman primate meals differed between the three habitat types (F = 201.273, p < 0.001): it was significantly higher in lowland forest than in gallery forest (Bonferroni posthoc test p < 0.001) and significantly higher in gallery forest than in savanna (Bonferroni posthoc test p < 0.001) (Figure 2).
Data were also examined for evidence of direct contact with nonhuman primate blood and body fluids. Two types of evidence for direct exposure were examined: self-reports of scratches or bites from live nonhuman primates and self-reports of injuries involving body fluids associated with hunting and butchering nonhuman primates (Table 2). Injuries associated with hunting and butchering occurred in 14 of the 17 villages and in all three habitation types, with a total of 1.67% of participants reporting such injuries (Appendix Table). Scratches or bites from nonhuman primates occurred in 14 villages in gallery forest and forest but did not occur in savanna sites. A total of 2.64% of participants reported such injuries. Of the participants who reported direct contact with nonhuman primate blood and body fluids through scratches, bites, or injuries, 91.2% reported butchering nonhuman primates, 73.0% reported hunting nonhuman primates, and 43.1% reported keeping a nonhuman primate as a pet. Men made up 82.5% of participants reporting direct contact, and women made up 17.5%. Most reports of direct contact involved monkeys (73.7%), although some direct contact was reported with gorillas (16.7%) and chimpanzees (9.6%).
Hunting nonhuman primates is a biologically ancient behavior that we share with our closest living relatives, the chimpanzees (24). Human hunting techniques and patterns, however, have changed substantially in contemporary times. During the 20th century, firearms increased the efficiency and frequency of hunting. Both subsistence and commercial hunting with wire snares and firearms are widespread activities throughout the forests of central Africa (1,25,26). In addition, road networks and increasing opportunities for transporting hunted game have led to an increase in sales and the rate of hunting (27).
Hunting and butchering nonhuman primates has been linked to the emergence of infectious disease (1,4). Hunting a red colobus (Procolobus badius oustaleti) has been implicated in a localized epidemic of monkeypox that continued for four generations of human-to-human contact (18). In addition, an outbreak of Ebola hemorrhagic fever in Mayibout, Gabon, in January 1996 was linked to butchering and eating a chimpanzee that had been found dead; 29 of 37 identified cases involved exposure to the chimpanzee (16). A number of subsequent epidemics in Gabon and Congo have also been linked to hunting and butchering apes (17).
Not only humans are at risk for diseases transmitted from nonhuman primates through hunting and butchering. Chimpanzees are regular hunters of monkeys and other forest vertebrates, and a study of Ebola hemorrhagic fever among chimpanzees in the Tai forests showed that the primary risk factor for contracting Ebola among wild chimpanzees was hunting behavior, which showed a stronger association with infection than other acknowledged risk factors, such as “touching dead bodies” (28).
More recent research suggests that hunting and butchering nonhuman primates resulted in the emergence of HIV (i.e., after cross-species transmission of SIV and subsequent spread) (21,22). While reconstructing the history of viral emergence is a substantial challenge, one possibility is that transmission of SIV associated with hunting and butchering is an ongoing process and that contemporary hunters may yet be found with SIV infection. This hypothesis has been strengthened by recent evidence suggesting that hunted nonhuman primates have a high rate of SIV infection (23) and evidence of hunting-associated cross-species transmission of another nonhuman primate retrovirus, simian foamy virus (19).
These results show that at least some rural villagers have a high level of exposure to nonhuman primates. While officially hunting of wild animals is forbidden, it is nonetheless widely accepted and permitted for personal use, so while the data may contain some bias, we do not feel that it is substantial. Our results show butchering to be the most common activity associated with contact with nonhuman primate blood and body fluids. More than 60% of the participants in the study reported having butchered a nonhuman primate, compared with ≈30% of participants who had hunted nonhuman primates. The higher frequency of persons reporting butchering as compared to hunting is expected, since those who hunt will often participate in some sort of butchering, generally including some preparation of wild game (e.g., disembowelment). Approximately 11% of the persons in the study reported keeping nonhuman primate pets. Because pets are usually young, the prevalence of chronic diseases in this population may be less than that among adult prey to which hunters and butchers are exposed. Nevertheless, because of the potential for regular contact with pet animals, even a low frequency of infections among pets may be important.
Villages from different habitats differed with regards to their reported exposure activities (Figure 2). Reported monthly consumption was significantly higher in the lowland forest sites. This finding may be due to the higher density and diversity of wildlife located close to these regions. Men and women both had high levels of contact with primate body fluids (Figure 3). While men were more likely than women to hunt wild animals, women were more likely than men to butcher. Because of differential participation in risk activities by men and women, these data suggest that gender-based interventions may be appropriate to decrease potential exposures to nonhuman primate blood and body fluids in central Africa.
The number of persons who reported direct contact with nonhuman primate blood or body fluids through scratches and bites from live primates or injuries during hunting and butchering was low. A total of 66 participants reported being in contact with nonhuman primate blood and body fluids through an injury associated with hunting or butchering wild game (Appendix Table). These persons often had multiple risk factors associated with nonhuman primate contact. Most of these persons reported a history of hunting nonhuman primates; eight persons reported only butchering. Results suggest that injuries associated with butchering may be less frequent than those associated with hunting, although injuries associated with butchering may be less severe and therefore less memorable than injuries associated with hunting and therefore underreported.
The results of this study show that contact with nonhuman primates, through both keeping wild animal pets and hunting and butchering nonhuman primates, is not confined to a small segment of the rural population. Rural central Africans are more highly exposed to microorganisms present in nonhuman primates than was previously considered. Persons in industrialized countries who have regular contact with nonhuman primates, such as laboratory workers, are known to risk contracting infectious diseases from nonhuman primates, such as herpesvirus B, SIV, and simian foamy virus. These occupationally exposed persons are the subject of extensive public health interventions, aimed at controlling zoonotic transmission (29,30). While poor rural villages depend on wild game for animal protein, education on the risks associated with contact with nonhuman primate blood is an essential step for these communities, as is work to develop economic alternatives to hunting. Studies in such villages can provide further insights into behavioral links with disease emergence. Behavioral interventions aimed at decreasing exposure to nonhuman primates in villages with high exposure rates may provide an opportunity to prevent both zoonosis on an individual level, as well as emergence events that have the potential for global effects.
Dr. Wolfe is an assistant professor in the Departments of Epidemiology and Molecular Microbiology and Immunology at the Johns Hopkins Bloomberg School of Public Health. He studies the ecology and evolution of infectious diseases in Africa and Southeast Asia, with a particular focus on the interface between humans and nonhuman primates.
Acknowledgments
We thank the government of Cameroon for permission to undertake this work; A. Boupda, K Long, the EICAM Project Staff, and the U.S. Embassy in Yaounde for assistance in carrying out the work; and W. Sateren and T. Folks for valuable comments.
This work was supported by an award from the U.S. Military HIV Research Program (to D.B.); an International Research Scientist Development Award from the National Institutes of Health, Fogarty International Center (K01 TW00003-01 to N.W.); and an award from the Johns Hopkins Bloomberg School of Public Health, Center for a Livable Future (to A.P. and N.W.).
The views and opinions expressed herein do not necessarily reflect those of the U.S. Army or the Department of Defense.
References
- Wolfe NW, Mpoudi-Ngole E, Gockowski J, Muchaal PK, Nolte C, Prosser AT, Deforestation, hunting and the ecology of microbial emergence. [serial on the Internet]. Glob Change Hum Health. 2000;1:10–25 Available from http://ipsapp008.kluweronline.com/IPS/content/ext/x/J/4731/I/1/A/4/abstract.htm#. DOIGoogle Scholar
- Ruch TC. Diseases of laboratory primates. Philadelphia: W.B. Saunders Company; 1959.
- Brack M. Agents transmissible from simians to man. Berlin: Springer-Verlag; 1987.
- Wolfe ND, Escalate AA, Karesh WB, Kilbourne AM, Spielman A, Lal AA. Wild primate populations in emerging infectious disease: the missing link? Emerg Infect Dis [serial on the Internet]. 1998 Apr–Jun [cited 1998 May 28]. Available from https://wwwnc.cdc.gov/eid/article/4/2/98-0202_article
- Wallis J, Lee DR. Primate conservation: the prevention of disease transmission. Int J Primatol. 1999;20:803–26. DOIGoogle Scholar
- Heneine W, Switzer WM, Sandstrom P, Brown J, Vedapuri S, Schable CA, Identification of a human population infected with simian foamy viruses. Nat Med. 1998;4:403–7. DOIPubMedGoogle Scholar
- Huff JL, Barry PA. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg Infect Dis [serial on the Internet]. 2003 Feb [cited 2003 Jan 13]. Available from https://wwwnc.cdc.gov/eid/article/9/2/02-0272_article
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Washington: U.S. Government Printing Office; 1997.
- Kalter SS, Millstein CH, Boncyk LH, Cummins LB. Tuberculosis in nonhuman primates as a threat to humans. Dev Biol Stand. 1978;41:85–91.PubMedGoogle Scholar
- Graczyk TK, Mudakikwa AB, Cranfield MR, Eilenberger U. Hyperkeratotic mange caused by Sarcoptes scabiei (Acariformes: Sarcoptidae) in juvenile human-habituated mountain gorillas (Gorilla gorilla beringei). Parasitol Res. 2001;87:1024–8.PubMedGoogle Scholar
- Sleeman JM, Meader LL, Mudakikwa AB, Foster JW, Patton S. Gastrointestinal parasites of mountain gorillas (Gorilla gorilla beringei) in the Parc National des Volcans, Rwanda. J Zoo Wildl Med. 2000;31:322–8.PubMedGoogle Scholar
- Butynski TM, Kalina J. Gorilla tourism: a critical look. In: Milner-Gulland EJ, Mace R. editors. Conservation of biological resources. Oxford: Blackwell Science; 1998. p. 294–366.
- Khabbaz RF, Heneine W, George JR, Parekh B, Rowe T, Woods T, Brief report: infection of a laboratory worker with simian immunodeficiency virus. N Engl J Med. 1994;330:172–7. DOIPubMedGoogle Scholar
- Shah KV. A review of the circumstances and consequences of simian virus SV40 contamination of human vaccines. Symposium on Continuous Cell Lines as Substrates for Biologicals. Developments in biological standardization, Vol. 70; 1989.
- Renquist DM, Whitney RA. Zoonoses acquired from pet primates. Vet Clin North Am Small Anim Pract. 1987;17:219–40.PubMedGoogle Scholar
- World Health Organization. Outbreak of Ebola haemorrhagic fever in Gabon officially declared over. Wkly Epidemiol Rec. 1996;71:125–6.
- World Health Organization. Outbreak(s) of Ebola haemorrhagic fever, October 2001–July 2002. Wkly Epidemiol Rec. 2003;78:223–8.PubMedGoogle Scholar
- Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–12.PubMedGoogle Scholar
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, Naturally acquired simian retrovirus infections among central African hunters. Lancet. 2004;363:932–7. DOIPubMedGoogle Scholar
- Le Guenno B, Formentry P, Wyers M, Gounon P, Walker F, Boesch C. Isolation and partial characterization of a new strain of Ebola virus. Lancet. 1995;345:1271–444. DOIPubMedGoogle Scholar
- Wrangham R, Wilson M, Hare B, Wolfe ND. Chimpanzee predation and the ecology of microbial exchange. Microb Ecol Health Dis. 2000;12:186–8. DOIGoogle Scholar
- Hahn BH, Shaw GM, DeCock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–14. DOIPubMedGoogle Scholar
- Peeters M, Courgnaud V, Abela B, Auzel P, Pourrut X, Bibollet-Ruche F, Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg Infect Dis [serial on the Internet]. 2002 May [cited 2002 March 1]. Available from https://wwwnc.cdc.gov/eid/article/8/5/01-0522_article
- Noss AJ. The impacts of cable snare hunting on wildlife populations in the forests of the Central African Republic. Conserv Biol. 1998;12:390–8. DOIGoogle Scholar
- Fa JE, García-Yuste JE. Commercial bushmeat hunting in the Monte Mitra forests, Equatorial Guinea: extent and impact. Anim Biodivers Conserv. 2001;24:31–52.
- Wilkie DS, Sidle JG, Boundzanga GC. Mechanized logging, market hunting and a bank loan in Congo. Conserv Biol. 1992;6:570–80. DOIGoogle Scholar
- Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d’Ivoire. J Infect Dis. 1999;179(Suppl 1):S120–6. DOIPubMedGoogle Scholar
- Richardson JH. Basic considerations in assessing and preventing occupational infections in personnel working with nonhuman primates. J Med Primatol. 1987;16:83–9.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 12—December 2004
| EID Search Options |
|---|
|
|
|
|
|
|
![Thumbnail of Percentage of male and female participants reporting exposure to wild game taxa (gorilla [G], chimpanzee [C], monkey [M], and rodent [R]) through keeping pets, hunting, butchering, and eating.](/eid/images/04-0062-F3-tn.gif)
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nathan D. Wolfe, Johns Hopkins University, Bloomberg School of Public Health, Central Africa Program, 624 N. Broadway #217, Baltimore, MD 21205, USA; fax: 410-502-0530
Top