Volume 10, Number 7—July 2004
Perspective
Environmental and Occupational Health Response to SARS, Taiwan, 2003
Abstract
The Taiwan Department of Health requested assistance from the Centers for Disease Control and Prevention (CDC) in controlling an outbreak of severe acute respiratory syndrome (SARS). The CDC SARS response team included industrial hygiene specialists from the National Institute for Occupational Safety and Health (NIOSH). Four NIOSH specialists visited hospitals and medical centers throughout Taiwan and assisted with designing and evaluating ventilation modifications for infection control, developed guidelines for converting hospital rooms into SARS patient isolation rooms, prepared designs for the rapid conversion of a vacated military facility into a SARS screening and observation facility, assessed environmental aspects of dedicated SARS hospitals, and worked in concert with the Taiwanese to develop hospital ventilation guidelines. We describe the environmental findings and observations from this response, including the rapid reconfiguration of medical facilities during a national health emergency, and discuss environmental challenges should SARS or a SARS-like virus emerge again.
The outbreak of severe acute respiratory syndrome (SARS) placed unprecedented demands on healthcare practitioners, healthcare institutions, and public works personnel worldwide. Taiwan reported the third largest number of SARS infections and deaths, followed by Hong Kong and mainland China (1). At the request of the Taiwan Department of Health, the Centers for Disease Control and Prevention (CDC) sent staff to Taiwan that included epidemiologists, infectious disease experts, and environmental and logistical specialists. Industrial hygienists were requested to investigate and help develop guidance for hospitals about patient isolation rooms, personal protective equipment, general infection control, and hospital health and safety.
From April 29, 2003, through June 13, 2003, four industrial hygienists from the National Institute for Occupational Safety and Health (NIOSH) conducted environmental assessments of 32 hospitals and medical centers throughout Taiwan. NIOSH staff were deployed serially (a team of two initially and later, two persons at two different times) within larger teams stationed first in Taipei and then in Kaohsiung. CDC personnel worked together with Taiwanese scientists from the Taiwan Department of Health (DOH), Taiwan Center for Disease Control, National Taiwan University, College of Public Health (NTUCPH), and the Taiwan Institute of Occupational Safety and Health (Taiwan IOSH). During the peak of the SARS epidemic, CDC environmental support focused on immediate steps to isolate SARS patients, protect healthcare workers and other personnel during fever screening and patient care, and provide advice on disinfection, direct contact, and airborne precautions. As the epidemic waned, efforts turned to assessing the implementation of infection control practices, strategies for handling future SARS patients, facility designs for effective patient isolation and fever screening stations, personal protective equipment practices, and training of healthcare workers. Thirty-two hospitals or medical centers that were either accepting and treating SARS patients or were under consideration for use as dedicated SARS treatment facilities in anticipation of a more widely disseminated epidemic were visited.
We describe an uncharacteristic industrial hygiene and public health response that occurred under conditions of a national health emergency. The circumstances, an evolving epidemic occurring in a worldwide atmosphere of tremendous uncertainty, elicited a unique response, which went beyond the traditional industrial hygiene investigative model. The requirement that the response teams deploy to the field on short notice, swiftly conduct multiple site investigations, and provide expedient recommendations on multiple topics in various healthcare environments, increased the challenges of this assignment. Although environmental findings and observations from hospital site visits and the rapid reconfiguration of medical facilities into dedicated SARS patient facilities are described, they are not necessarily prescriptive. Global health emergencies demand quick action, which was the case for the SARS epidemic. We describe the environmental challenges that could occur should SARS or a SARS-like virus emerge again. Prescriptive environmental guidelines are available elsewhere (2). Comprehensive ventilation engineering or facility evaluations of every hospital or healthcare facility were not possible to obtain.
Two staff members arrived April 29, 2003, and met with CDC team members to coordinate response roles. Meetings were arranged with Taiwanese government officials and scientists, including Taiwan DOH, Taiwan Centers for Disease Control, NTUCPH, and Taiwan IOSH. The need was agreed on for controls to isolate SARS patients and ensure protection of healthcare workers and other personnel exposed to patients suspected or known to be infected with SARS. A four-phase environmental approach was developed, which included the following: 1) conduct environmental needs assessments for healthcare facilities; 2) conduct environmental field assessments for healthcare facilities; 3) develop written environmental guidelines for healthcare facilities; and 4) conduct environmental audits of selected healthcare facilities.
Environmental Needs Assessment for Healthcare Facilities
In collaboration with Taiwanese scientists, a needs assessment for healthcare facilities was conducted to understand the most important environmental issues for controlling the spread of SARS in hospitals. Additional airborne infection isolation rooms (AIIRs) were needed on a temporary and a permanent basis. Developing environmental guidelines for design and evaluation of such rooms was a priority. Training healthcare workers in appropriate use of personal protective equipment and infection control practices was also an immediate need at the hospital level.
Environmental Field Assessments of Healthcare Facilities
Field assessments of healthcare facilities were conducted to better understand standard design and ventilation parameters and how existing AIIRS were configured. The Taiwan Center for Disease Control selected the facilities to represent a cross-section of national, municipal, military, and private hospitals. Environmental field teams typically included NIOSH specialists, a Taiwan Center for Disease Control physician, an occupational health specialist, a Taiwan IOSH engineer, and a doctoral student from NTUCPH. Discussions were held with senior hospital management to understand general hospital configurations, total patient and AIIR capacity, infection control practices, and to determine if SARS patients were being treated at the facility. Ventilation designs were discussed with facilities staff, ventilation drawings were reviewed, and AIIRS, if present, were observed. Discussions with staff during the site visit allowed the team to evaluate environmental issues regarding the expedient addition of isolation rooms, issues affecting infection control, and the selection and use of appropriate personal protective equipment. Table 1 describes the first 10 hospitals and 2 fever clinics that were visited from April 29, 2003, through May 13, 2003, in and around Taipei and Kaoshiung, Taiwan.
Environmental Guidelines for Healthcare Facilities
A Taiwan IOSH engineer, 27 Taiwanese public health and ventilation specialists, and a NIOSH industrial hygiene specialists developed Guidelines for the Integrity and Inspection of SARS Isolation Wards. The information was to be used to construct permanent infrastructure and included the appropriate layout of a SARS treatment room and ward, ventilation design for sufficient negative pressure in AIIRs, and filtration and treatment of exhausted air. This document also included measures to enhance infection control and protect healthcare workers during maintenance of the isolation room and ventilation system. The document was written in Chinese and was posted on Web sites of the Taiwan Center for Disease Control and IOSH and distributed to healthcare facilities (http://www.iosh.gov.tw/eversion/sarse.htm). The Taiwan Center for Disease Control also used this document in training sessions for healthcare managers and workers throughout Taiwan.
Because of an immediate need for simple ventilation modifications to reconfigure existing patient rooms to serve as AIIRs, NIOSH staff developed a list of possible modifications to create and confirm negative pressure in typical patient rooms. Applications varied by facility and included increasing exhaust air volume with the addition of assist fans, ways to reduce room leakage and confirm negative pressure, such as using flutter strips on doors or pressure gauges on walls. More complex modifications that used self-contained air exchange, HEPA filtration, and ventilation units were also included.
Environmental Audits of Selected Healthcare Facilities
Audits evaluated how well the SARS isolation ward guidelines had been implemented. Hospital managers were contacted, and walkthrough surveys were arranged for investigators. The general objectives were to determine the adequacy of AIIRs, provide guidance for construction or conversion of existing patient rooms into AIIRs, provide technical assistance on ventilation and other controls (e.g., isolation, procedures, and training), evaluate appropriate use of personal protective equipment, and provide guidance on various environmental considerations for infection control.
Kaohsiung County, Tainan, and Chai-Yi
Twelve hospitals and medical centers in southern Taiwan were visited May 17–25, 2003. Meetings were held with facilities and ventilation engineers, hospital administrators, and medical and nursing staff to understand the number, type, and location of AIIRs. Mechanical drawings, blueprints, and ventilation testing reports were reviewed, and walkthrough surveys were conducted. Visible smoke testing was performed to evaluate pressurization between nurses’ stations and SARS patients’ wards and for as many individual patient rooms as possible. When possible, air handling units, HEPA filters, outside air intakes, and rooftop exhaust ductwork were inspected. Infection control procedures and personal protective equipment use were reviewed, including availability, staff knowledge regarding proper use, and implementation.
Standard versus simple negative-pressure isolation rooms were observed. Typically, standard isolation rooms had an anteroom for use by healthcare workers to put on and take off personal protective equipment, a digital or analog pressure manometer mounted outside the door, hard rather than suspended ceilings, and walls that extended floor-to-ceiling. Headwall and utility penetrations were sealed to reduce leakage, maintain negative pressure, and control airflow. Constant volume air-handling units were commonly used and configured to operate in the single pass mode. Most air-handling units could be tested and balanced to maintain negative pressure of 2.5 to 20 pascal (0.008–0.08 inches of water). Exhaust for standard AIIRs were often configured with HEPA filtration. Some facilities installed ultraviolet germicidal irradiation (UVGI) lamps in HEPA filter banks, although the efficacy of using UVGI for control of SARS-associated coronavirus was uncertain.
Most of the simple isolation rooms had been designed for isolating patients with infectious diseases such as scabies. These rooms lacked an anteroom, generally had window-mounted air-conditioning units, and small (1,000–2,000 ft3/min) window-mounted vane axial fans for negative pressure. Wall penetrations were typically not sealed, manometers were generally not present, and ceilings were not hard. Unfiltered room air was exhausted outdoors through windows.
Aggressive infection control measures were evident in all hospitals. Hand sanitation stations with automated dispensers were abundant, especially on nurses’ wards, at elevator landings, and at every hospital entrance. Infection control staff dispensed sanitizing gels and cloth or disposable hand-cleaning wipes. Infection control personnel were also stationed at hospital entrances and screened for fever by measuring forehead skin temperatures. One hospital installed forward-looking infrared scanning cameras that displayed temperatures on TV monitors next to nurses’ stations. Visitors or staff with fever were denied entrance and sent to fever clinics outside the hospital for medical follow-up. Healthcare workers, hospital staff, and visitors all wore filtering face-piece surgical masks or respirators of varying brands and efficiencies (N95 to N100). Most hospitals cordoned a gurney pathway from ambulance entrances to an elevator landing, where a designated and preprogrammed elevator transported SARS patients to SARS wards. When meetings were conducted, all participants wore respirators or masks, and handshaking was minimized or discouraged.
Appropriate protective equipment (including eye protection, protective suits, aprons, gloves, head and foot coverings, and respirators) was widely available and healthcare workers were knowledgeable about their use. In many nurses’ stations, posters describing standard operating procedures for SARS protective equipment were present. Environmental findings were discussed at a closing meeting, and written reports were later sent to each hospital. Table 2 summarizes general environmental aspects of 10 hospitals investigated in southern Taiwan and provides examples of recommendations provided to these hospitals.
Kaohsiung SARS Screening and Observation Facilities
Two proposals for the construction of specialized SARS screening and isolation facilities in southern Taiwan were reviewed by NIOSH and Taiwanese environmental specialists. One proposal considered configuring arrays of shipping containers (widely available in this port city) into patient AIIRs linked in hub-and-spoke fashion by a central nurses’ station. This proposal was not recommended because of uncontrolled solar loads on the containers and feasibility issues for patient emergency medical treatment procedures. The second proposal was to convert vacated military barracks into a SARS patient–screening facility. An ambitious timeline required converting an open bay barracks into 20 individual patient isolation rooms and ultimately converting another barracks of similar design for a total of 40 beds. Demolition and reconstruction were to be completed and a functional facility available within 48 hours. A meeting was held May 22, 2003, to tour the site, sketch a preliminary design, and provide verbal recommendations for a proposed redesign of the open bays into an 18-bed facility, configured into simple patient isolation rooms. Demolition began immediately. The responders provided a detailed guidance document the following day, which outlined the following areas: facility design, construction, and renovation specifications for patient rooms; ventilation specifications, including hood designs to improve axial exhaust fans; fire, safety, and environmental guidelines; infection control practices for patients and healthcare workers; placement of sanitary facilities; and patient and staff traffic flow through the facility.
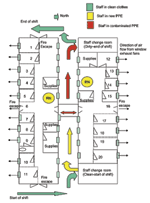
This facility was dedicated on May 28, 2003, substantially increasing the number of AIIRs available in southern Taiwan. The facility could also function as a quarantine station if needed. The floor plan of this converted barracks building is shown in Figure 1.
Kaohsiung County, Taidong, Taichung, Hualien, and Taipei
The Taiwan DOH issued a directive on April 23, 2003, mandating the establishment of 11 dedicated SARS hospitals and medical centers geographically dispersed throughout Taiwan (Table 3). General care hospitals were directed to treat mild SARS patients (those not on ventilators and with substantial pulmonary capacity) and patients undergoing rehabilitation; patients who needed ventilation were sent to medical centers with intensive care units. SARS-designated hospitals ceased providing general patient care and began complying with the AIIR construction schedule, including reengineering or installation of new ventilation equipment. After the design phase, hospitals completed the conversion (start of construction to patient acceptance) on an average of 13 days, resulting in a total of 698 negative-pressure AIIRs constructed by the completion of the nationwide project.
From May 31 through June 10, 2003, a total of 11 dedicated SARS hospitals were evaluated to assess AIIRs and wards, infection control practices, healthcare worker and patient entrance and egress pathways, protective equipment practices, and healthcare worker training (Figure 2). As part of the arrangement by the Taiwan Center for Disease Control for the hospital site visits, hospital management were given a questionnaire that requested design criteria, number of isolation rooms, personal protective equipment requirements, and ventilation specifications. Evaluations began with an opening conference with administrative, engineering, infection control, and healthcare worker staff. Hospital objectives and the status of the hospital modifications were discussed, blueprints were reviewed, ventilation system and AIIR design were discussed, healthcare worker training was reviewed, and the personal protective equipment protocol was observed. A walkthrough of the patient and healthcare worker pathway was conducted, including an inspection of the various isolation gradients (nurses’ stations, change rooms, isolation ward corridor, anterooms, and AIIRs). Visible smoke was used to evaluate the pressurization of AIIRs and to assess airflow patterns both within the IR and the ventilation zones and the ventilation system (supply air location, exhaust discharge, HEPA filters, and UVGI) was inspected. Maintenance practices, establishment of an infection control department, location of hand-washing stations, room pressure monitors, and availability of personal protective equipment were reviewed. Closing meetings were held with hospital management and healthcare worker staff to review findings and recommendations and followed up with written reports.
The Taiwan Industrial Technology Research Institute, Taiwan IOSH, and Taiwan Center for Disease Control provided general facility design specifications for these hospitals and other general care facilities treating SARS patients. Most hospitals contracted with architectural and engineering firms to manage design and reconstruction. A national hospital steering committee was formed to guide this effort. Design criteria included specifications from existing CDC tuberculosis guidelines (3), the American Society of Heating, Refrigerating and Air-Conditioning Engineers Design Manual for Hospitals and Clinics (4), and the American Institute of Architects Guidelines for Hospitals and Health Care Facilities (5).
Massive infrastructure modifications were necessary to achieve the desired objectives of increased capacity to triage and treat seriously ill patients and at the same time protect healthcare workers. Examples include the reconfiguration of the entrance and egress corridors for healthcare workers and patients to ensure complete physical and airflow separation, establish multistep, interlocked ventilation zones for healthcare workers to put on the required personal protective equipment, as well as a similarly tiered degowning procedures with final shower-out. Patients were received in a designated buffer area (in some cases containing automatic-spray cleaning systems to sanitize between patients) with dedicated elevators and corridors for patient flow. All negative-pressure isolation rooms were designed with single-pass (100% outside air with no recirculation) dedicated exhaust systems. Exhaust air in all hospitals was treated with HEPA filtration and UVGI before discharge. Visual indicators at the IR door and a remote indicator panel in the nurses’ station monitored isolation room pressure. Isolation design included a pressure gradient from the clean (e.g., nurses’ station or change room) to the less clean (patient room), including buffer zones to achieve the desired conditions (Figure 3). Designated laundry and medical waste corridors were established, and aggressive cleaning regimens were implemented to ensure frequent sanitation of all areas, including twice daily cleaning of patient rooms and autoclaving of waste before its removal from the facility. Personal protective equipment requirements varied somewhat among hospitals, but typically healthcare workers wore an N95 or N100 respirator, protective suit, double or triple disposable gloves, shoe covers, outer gown, hair cover or hood, and face shield, goggles, or both (Figure 4). To help healthcare workers alleviate heat stress from the encumbering personal protective equipment, work shifts in some hospitals were reduced to 3 or 4 hours. All hospitals had an established infection control department and an infection control plan. A summary of the key design features of the 11 SARS-dedicated hospitals is listed in Table 4.
Although the major reconfiguration design goals were the same for all hospitals, structural realities and other practical considerations resulted in differences in final configuration. Design changes during construction were also necessary to overcome unforeseen engineering obstacles. Examples of differences include the following: 2 of 11 hospitals did not have elevator capability for healthcare workers to access isolation wards, 5 of 11 hospitals did not have positive-pressure nurses’ stations in the isolation ward, not all hospitals had anterooms, and 3 of 11 hospitals did not have complete patient and healthcare worker pathway separation. Other differences included the number of UVGI units in the ventilation system, whether the UVGI was located in front of or behind the HEPA or prefilter, the type (electronic or mechanical) and location of room pressure monitors, the redundancy strategy (dual exhaust, dual filter, or both), and whether the ventilation system received testing and balancing after installation.
From a facility, personnel, environmental and occupational health perspective, the response to SARS in Taiwan had a profound impact on the healthcare system of the nation. The Taiwanese government responded in a swift and comprehensive manner to contain the outbreak. Although major gaps in knowledge existed regarding this first emerged infectious disease of the 21st century, decisions involving massive resource commitments had to be made quickly and decisively. Hospitals and medical centers islandwide renovated their facilities rapidly or constructed new patient treatment facilities to contain and treat known or suspected SARS patients. Healthcare workers learned to use personal protective equipment in a far more judicious and extensive manner than they were accustomed to. Large-scale retraining and reassignment of thousands of healthcare personnel was also required.
When SARS or a SARS-like pandemic recurs, industrial hygiene specialists will be faced with similar circumstances and should anticipate that they will be forced to respond in a highly charged environment of considerable scientific uncertainty. While the standard industrial hygiene rubrics of anticipation, recognition, evaluation, and control remain useful, more inventive approaches to risk and hazard assessment will be necessary and will test responders’ capabilities and tenacity. Some of the challenges these environmental specialists encountered during the SARS response included the following: developing expedient guidelines for engineering and administrative controls for workers and workplaces; developing personal protective equipment use guidelines for healthcare workers and the general public, including questions regarding the feasibility of disinfection and reuse and of disposable respirators; developing and providing training on personal protective equipment use by workers, especially healthcare workers; evaluating hospital isolation rooms and ventilation systems, including containment of window air-conditioner condensate from SARS patient rooms; working with hospital infection control and facilities personnel to develop alternative triage facilities, such as fever screening clinics; advising facilities design personnel on hospital reconfiguration to improve patient transport and patient isolation; developing, applying, and interpreting results from unvalidated and novel environmental sampling techniques; creating effective risk communication tools for workers and the general public; advising local officials on issues of isolation, quarantine, and other public safety concerns, including obtuse inquiries such as the utility of disinfecting septic systems contaminated with SARS-CoV, and evaluating the feasibility of novel patient containment and treatment facilities, and the use of unproven, yet theoretically reasonable control technologies in an emergency situation.
Dr. Esswein is a commissioned officer in the U.S. Public Health Service assigned as a senior industrial hygienist with the Denver Field Office, NIOSH, CDC. His research interests include indoor environmental quality, occupational hygiene in developing countries, and development of dermal exposure assessment and removal techniques for the presence of lead.
Acknowledgment
We thank Daniel B. Jernigan for his excellent leadership and guidance of the CDC team during the initial weeks of the Taiwan SARS response; Susan A. Maloney for her timely advice and support of the hospital evaluation project; Ta-Yuan Chang for his role in the initial visits to hospitals in and around Taipei, Taiwan, as well as the future training of others to perform environmental sampling for the SARS virus; Chang-Chuan Chan for facilitating the environmental evaluation of healthcare facilities and his scientific input to the development of the four-phased approach described in this article; William Wong, Peter B. Bloland, and Sarah Y. Park for their contributions, leadership, and support; Howard W.-H. Hsiao for his support conducting hospital site visits, with translations in the field, and for his overall sense of equanimity; Frank Chin-Hsun Su for his enthusiasm, support, and energy during the design and construction of the Kaohsiung SARS Screening and Observation Facility; and Ching-Chu Chiang for his outstanding support and kindness.
References
- Centers for Disease Control and Prevention. Use of quarantine to prevent transmission of severe acute respiratory syndrome: Taiwan, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:680–3.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Guidelines for environmental infection control in health care facilities, 2003. [accessed 23 Oct 2003]. Available from: www.cdc.gov/ncidod/hip/enviro/guide.htm
- Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Morb Mortal Recomm Rep. 1994;43(RR-13):65.
- American Society of Heating. Refrigerating and Air-Conditioning Engineers, Inc. HVAC design manual for hospitals and clinics, ASHRAE Special project 91, 2003. Atlanta: ASHRAE; 2003.
- American Institute of Architects and Facilities Guidelines Institute. Guidelines for design and construction of hospital and healthcare facilities. Washington: American Institute of Architects Press; 2001.
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 7—July 2004
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Eric J. Esswein, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Denver Field Office, Denver, CO 80225-0226, USA; fax: 303-236-6072
Top