Volume 10, Number 9—September 2004
Research
Detection of Anaplasma phagocytophilum DNA in Ixodes Ticks (Acari: Ixodidae) from Madeira Island and Setúbal District, Mainland Portugal
Abstract
A total of 278 Ixodes ticks, collected from Madeira Island and Setúbal District, mainland Portugal, were examined by polymerase chain reaction (PCR) for the presence of Anaplasma phagocytophilum. Six (4%) of 142 Ixodes ricinus nymphs collected in Madeira Island, and 1 nymph and 1 male (2%) of 93 I. ventalloi collected in Setúbal District tested positive for A. phagocytophilum msp2 genes or rrs. Infection was not detected among 43 I. ricinus on mainland Portugal. All PCR products were confirmed by nucleotide sequencing to be identical or to be most closely related to A. phagocytophilum. To our knowledge, this is the first evidence of A. phagocytophilum in ticks from Setúbal District, mainland Portugal, and the first documentation of Anaplasma infection in I. ventalloi. Moreover, these findings confirm the persistence of A. phagocytophilum in Madeira Island’s I. ricinus.
Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila, E. equi, and the human granulocytic ehrlichiosis agent [HGE agent] [1]) is well established as a worldwide tickborne agent of veterinary importance and is considered an emerging human pathogen. The initial reports of human disease caused by A. phagocytophilum, now called human granulocytic anaplasmosis, came from Minnesota and Wisconsin in 1994 (2,3). Human granulocytic anaplasmosis is an acute, nonspecific febrile illness characterized by headache, myalgias, malaise, and hematologic abnormalities, such as thrombocytopenia and leukopenia as well as elevated levels of hepatic transaminases (4). Since that first report, an increasing number of cases have been described, mostly in the upper Midwest and in the Northeast regions of the United States (5). Three years later, in 1997, acute cases of this disease were also described in Europe (6,7). Several serologic and polymerase chain reaction (PCR)-based studies described the wide distribution of A. phagocytophilum across Europe and in some parts of the Middle East and Asia (8–10). Nevertheless, confirmed cases of human granulocytic anaplasmosis are rare; most European cases are described in Slovenia (11), with only a few reports from other European countries (12) and China (13).
The ecology of A. phagocytophilum is still being defined, but the agent is thought to be maintained in nature in a tick-rodent cycle, similar to that of Borrelia burdgdorferi (the agent of Lyme disease), with humans being involved only as incidental “dead-end” hosts (14–17). Exposure to tick bites is considered to be the most common route of human infection, although human granulocytic anaplasmosis has been reported after perinatal transmission or contact with infected animal blood (18,19). A. phagocytophilum is associated with Ixodes ticks that are known vectors, including I. scapularis, I. pacificus, and I. spinipalpis in the United States (15,20,21), I. ricinus mostly in southern, central and northern European regions (22–26), I. trianguliceps in the United Kingdom (27), and Ixodes persulcatus in eastern parts of Europe (28) and Asia (9).
In Portugal little information is available concerning the epidemiology of A. phagocytophilum; the agent was documented only once in I. ricinus ticks from Madeira Island (Núncio MS, et al, unpub data). However, the true prevalence and public health impact of A. phagocytophlum is likely underestimated since little research has been conducted on this bacterium in Portugal. In fact, seasonal outbreaks of enzootic abortions and unspecific febrile illness (commonly named pasture fever) in domestic ruminants, which could be attributable to A. phagocytophilum, have been known to breeders and veterinarians across the country for years. Thus, to expand knowledge of A. phagocytophilum in Portugal, a detailed investigation was initiated. The preliminary results concerning agent distribution are presented here. The purpose of this study was to investigate both the persistence of A. phagocytophilum on Madeira Island, where it was initially described, and the presence of the agent in Ixodes ticks from mainland Portugal.
Tick Sampling
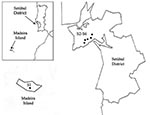
During 2003 and the beginning of 2004, adults and nymphs were collected from one site on Madeira Island (site 1, Paúl da Serra–Porto Moniz) and from five different sites in the Setúbal District, mainland Portugal (site 2, Barris–Palmela; site 3, Baixa de Palmela; site 4, Picheleiros–Azeitão, site 5, Azeitão, site 6, Maçã–Sesimbra) (Figure 1). Most ticks were unfed, actively questing arthropods; they were obtained by flagging vegetation on pastures and wooded areas bordering farms and country houses. In site 3, additional specimens were also collected from domestic cats (Felis catus domesticus). The ticks were identified by morphologic characteristics according to standard taxonomic keys (29,30).
Preparation of DNA Extracts from Ticks
Ticks were processed individually as described (25). Briefly, each tick was taken from the 70% ethanol solution used for storage, air dried, and boiled for 20 min in 100 μL of 0.7 mol/L ammonium hydroxide to free DNA. After cooling, the vial with the lysate was left open for 20 min at 90°C to evaporate the ammonia. The tick lysate was used directly for PCR. To monitor for occurrence of false-positive samples, negative controls were included during extraction of the tick DNA (one control sample for each six tick samples, with a minimum of two controls).
PCR Amplification
DNA amplifications were performed in a Biometra T-3 thermoblock thermal cycler (Biometra GmbH, Göttingen, Germany) with two sets of primers: msp465f and msp980r, derived from the highly conserved regions of major surface protein-2 (msp2) paralogous genes of A. phagocytophilum (31), and ge9f and ge10r, which amplify a fragment of the 16S rRNA gene of A. phagocytophilum (3). PCR was performed in a total volume of 50 μL that contained 1 μM of each primer, 2.5 U of Taq DNA polymerase (Roche, Mannheim, Germany), 200 μM of each deoxynucleotide triphosphate (GeneAmp PCR Reagent Kit, Perkin-Elmer, Foster City, CA), 10 mM Tris HCL, 1.5 mM MgCl2, and 50 mM KCl pH 8.3 (Roche), as described (3,31). Adult ticks were tested individually by using 5 μL of DNA extract. Nymphs were pooled according to geographic site, up to a maximum of 10 different tick extracts per reaction, and 10 μL of the pooled DNA was used for initial screening. All positive pools were confirmed in a second PCR round that used 5 μL of original DNA extract from each nymph. PCR products were separated on 1.5% agarose by electrophorectic migration, stained with ethidium bromide, and visualized under UV light. Quality controls included both positive and negative controls that were PCR amplified in parallel with all specimens. To minimize contamination, DNA preparation with setup, PCR, and sample analysis were performed in three separate rooms.
DNA Sequencing and Data Analysis
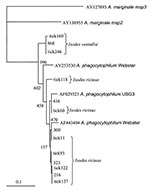
Each positive PCR product was sequenced after DNA purification by a MiniElute PCR Purification Kit (Qiagen, Valencia, CA). For DNA sequencing, the BigDye terminator cycle sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA), was used as recommended by the manufacturer. Sample amplifications were performed with the forward and reverse primers used for PCR identification (3,31), with the following modifications: 25 cycles of 96°C for 10 s, 4°C below the melting temperature of each primer for 5 s, and 60°C for 4 min. Dye Ex 96 Kit (Qiagen) was used to remove the dye terminators. Sequences were determined with a 3100 Genetic Analyzer sequencer (Applied Biosystems). After review and editing, sequence homology searches were made by BLASTN analysis of GenBank. Sequences were aligned by using ClustalX (32) with the neighbor-joining protocol and 1,000 bootstrap replications, and comparing with the 2 msp2 paralogs of A. phagocytophilum Webster strain (AY253530 and AF443404), one msp2 paralog of USG3 strain (AF029323), and with A. marginale msp2 (AY138955) and msp3 (AY127893) as outgroups. Dendrograms illustrating the similarity of msp2s were visualized with TreeView (33).
A total of 278 Ixodes ticks were tested for A. phagocytophilum DNA, including 142 I. ricinus from Madeira Island and 43 I. ricinus and 93 I. ventalloi from Setúbal District. The site of collection, origin, and tick stage are shown in Table 1 and Figure 1. PCR performed with the msp2 primers detected A. phagocytophilum DNA in seven pools of nymphs (six pools of 10 I. ricinus from site 1, Madeira Island, and one pool of 4 I. ventalloi from site 3, Setúbal District) and also in 1 male I. ventalloi from site 3, Setúbal District, as demonstrated by the characteristic 550-bp band. PCRs conducted on individual ticks that comprised positive pools confirmed the results and showed that only one nymph per positive pool contained A. phagocytophilum DNA (Tables 1 and 2). PCR test results were negative for all I. ricinus collected in the sites in Setúbal District. Overall, the infection rate was 6 (4%) of 142 for I. ricinus and 2 (2%) of 93 for I. ventalloi. Analysis based on direct amplicon sequencing showed the expected conserved 5′ end followed by ambiguous sequences that corresponded to the hypervariable central region of msp2, as anticipated based on the presence of >52 msp2 copies in the A. phagocytophilum HZ strain genome (34). Thus, for appropriate comparison and alignment, the msp2 5′ sequences were edited from the positions where unambiguous reads could be determined and terminated 70 nt into the sequence at the approximate beginning of the hypervariable region. A similar alignment protocol for the 3′ end of the msp2 amplicons showed more ambiguous positions, which prohibited effective alignment and sequence determination. Thus, msp2 sequence alignments depended upon approximately 70 nt 5′ to the hypervariable region and were performed less for phylogenetic stratification of A. phagocytophilum in the ticks than to confirm that the amplified msp2 sequences were not derived from other related Anaplasma or Ehrlichia spp. The nucleotide sequences determined for this 70-bp region amplified from all eight ticks showed 98.5%–85.7% similarity, 94.2%–86.9% similarity when compared to representative msp2 sequences of A. phagocytophilum Webster and USG3 strains, and 63.7%–35.0% similarity when compared to A. marginale msp2 and msp3 sequences (Figure 2). Sequences obtained from the two I. ventalloi from mainland Portugal clustered together and separately from other msp2 sequences obtained from I. ricinus on Madeira Island (Figure 2).
When amplified by using rrs primers ge9f and ge10r, compared to A. phagocytophilum U02521, sequences were 99% identical to two I. ventalloi (636/640 positions and 846/848 positions, respectively) on mainland Portugal and to three I. ricinus (836/841, 817/820, and 838/839 positions, respectively) on Madeira Island.
This study constitutes part of a larger effort to investigate the distribution of A. phagocytophilum in various regions of Portugal. Our data provide supporting evidence that A. phagocytophilum is present in actively questing I. ricinus from Madeira Island and in I. ventalloi from Setúbal District, mainland Portugal.
We used two approaches for identifying A. phagocytophilum in ticks: 1) standard amplification of rrs that can have limited sensitivity because of a single copy in each bacterial genome, and 2) amplification of msp2, a gene for which as many as 52 paralogs are present in the A. phagocytophilum genome and for which detection sensitivity is enhanced (34). The pitfall of msp2 amplification derives from targeting conserved sequences that flank a hypervariable central region, which results in amplicons with partial sequence ambiguity when cloning is not attempted before sequencing (31). These findings are highly unlikely to represent amplicon contamination since marked sequence diversity was observed, and since only a single tick from each pool was positive in each reaction. Although only limited data can gleaned by this analysis, which interrogates only nucleic acids of small size, Casey et al. have shown that msp2 “similarity” groups, reflecting clusters determined by a similar sequencing approach, can be useful in predicting phylogenetic relationships, particularly with reference to adaptation to specific host niches (35).
Madeira, the main island of the Madeira Archipelago, is located in the North Atlantic Ocean, about 800 km west of the African continent and 1,000 km from the European coast. On this island, I. ricinus is the most abundant tick species and the only Ixodes tick that was found in this study. A. phagocytophilum was detected in 4% of I. ricinus collected in Paúl da Serra. Our results corroborate previous findings, although prevalence here is slightly lower than the 7.5% infection rate in ticks previously collected in similar areas. These differences may be attributable to seasonal variations in A. phagocytophilum prevalence within reservoir hosts or ticks or to technical aspects of detection. Regardless, studies that use a greater number of samples and that are performed in different seasons, locations, and habitats will be needed to confirm the levels of infection. Nevertheless, these findings are generally similar to those described elsewhere in Europe, although prevalence rates can vary greatly with the origin of I. ricinus examined, ranging from a minimum of <1% in the United Kingdom, France, and Sweden (23,24,36) to a maximum of 24% to 29% in northern Italy, Germany, and Spain (22,25,26). The public health importance of these findings still remains to be determined. I. ricinus is an exophilic, three-host tick known to bite several domestic animals and humans in Portugal (30). Therefore, we can assume that the presence of A. phagocytophilum on Madeira Island I. ricinus suggests a potential health threat to animals and humans and should be investigated.
Although I. ricinus is not the main tick species in mainland Portugal, it can be found across the country in habitats with favorable conditions. Focused in Setúbal District, to the south of the Tejo River, our study detected I. ricinus in all five sites chosen for field work: Barris; Baixa de Palmela; Pischeleiros; Azeitão, and Maçã. In those sites, the distribution of I. ricinus was accompanied by another Ixodes species, I. ventalloi. Another ecologically interesting finding that should be further confirmed was that, although all of the I. ricinus from mainland Portugal tested negative, evidence of A. phagocytophilum was found in 2% of all I. ventalloi, including 5% collected in Baixa de Palmela. The msp2 sequences identified in these two ticks were more closely related to each other than to any msp2 sequence identified in ticks from Madeira Island. In contrast, A. phagocytophilum msp2 diversity in I. ricinus from Madeira Island was broad and showed overlap with gene sequences identified in North American strains, as observed for some A. phagocytophilum strains in the United Kingdom (35).
To our knowledge, this identification of A. phagocytophilum in ticks is the first from mainland Portugal and the first documentation of Anaplasma infection in I. ventalloi. This species is an endophilic, three-host tick well adapted to a broad range of habitats that vary from open, dry forest in semidesert Mediterranean areas to the mild humid conditions in the southern part of the British Isles. In Portugal, I. ventalloi infest a variety of small rodents, carnivores, and lizards but have not been found to feed on humans (30). A. phagocytophilum has already been reported in other ticks, besides the known vector species (37–41). The presence in alternate ticks is attributable to the existence of secondary maintenance cycles, in which A. phagocytophilum circulates between relatively host-specific, usually nonhuman-biting ticks and their hosts (38,39). Those additional cycles would buffer the agent from local extinction and help reestablish the primary cycles (38,39). Although this hypothesis might explain our results, the competency of I. ventalloi to act as vector for A. phagocytophilum has yet to be demonstrated. Moreover, the different average prevalences observed in each location suggest that A. phagocytophilum is not widely spread in ticks and that some reservoir animals or hosts are needed for its maintenance. Trapping and animal surveillance are needed to provide more information that could help to explain the biological importance of those findings.
Ms. Santos works at the Centre of Vector-borne and Infectious Diseases, National Institute of Health. Her research has been focused on tickborne agents, mainly rickettsial agents with human heath importance. She is pursuing a doctoral degree focused on human anaplasmosis and ehrlichiosis in Portugal.
Acknowledgments
We thank Maria Arminda Santos for useful help in field work and Sonia Pedro and Ricardo Jorge for valuable assistance with sequencing.
This research was partially supported by the Portuguese government through the Fundação para a Ciência e a Tecnologia grant BD/8610/2002 and through U.S. National Institutes of Health grant R01-AI41213 to J.S.D.
References
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65.PubMedGoogle Scholar
- Bakken JS, Dumler JS, Chen S-M, Eckman MR, Van Etta LL, Walker DH. Human granulocytic ehrlichiosis in the upper midwest United States, a new species emerging? JAMA. 1994;272:212–8. DOIPubMedGoogle Scholar
- Chen S-M, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic ehrlichia species as the etiologic agent of human diseases. J Clin Microbiol. 1994;32:589–95.PubMedGoogle Scholar
- Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. DOIPubMedGoogle Scholar
- Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554–60. DOIPubMedGoogle Scholar
- Petrovec M, Furlan SL, Zupanc TA, Strle F, Brouqui P, Roux V, Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–9.PubMedGoogle Scholar
- Lotric-Furlan S, Petrovec M, Zupanac TA, Nicholson WL, Sumner JW, Childs JE, Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin Infect Dis. 1998;27:424–8. DOIPubMedGoogle Scholar
- Keysary A, Amram L, Keren G, Sthoeger Z, Potasman I, Jacob A, Serologic evidence of human monocytic and granulocytic ehrlichiosis in Israel. Emerg Infect Dis. 1999;5:775–8. DOIPubMedGoogle Scholar
- Cao WC, Zhao QM, Zhang PH, Dumler JS, Zhang XT, Fang LQ, Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J Clin Microbiol. 2000;38:4208–10.PubMedGoogle Scholar
- Park JH, Heo EJ, Choi KS, Dumler JS, Chae JS. Detection of antibodies to Anaplasma phagocytophilum and Ehrlichia chaffeensis antigens in sera of Korean patients by western immunoblotting and indirect immunofluorescence assays. Clin Diagn Lab Immunol. 2003;10:1059–64.PubMedGoogle Scholar
- Lotric-Furlan S, Petrovec M, Avsic-Zupanc T, Strle F. Human granulocytic ehrlichiosis in Slovenia. Ann N Y Acad Sci. 2003;990:279–84. DOIPubMedGoogle Scholar
- Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–72. DOIPubMedGoogle Scholar
- Gao D, Cao W, Zhang X. Investigations on human ehrlichia infectious people in Daxingan Mountains [article in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:137–41.PubMedGoogle Scholar
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vector s and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. DOIPubMedGoogle Scholar
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD Jr, Dumler JS, Bakken JS, Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–12.PubMedGoogle Scholar
- Daniels TJ, Falco RC, Schwartz I, Varde S, Robbins RG. Deer ticks (Ixodes scapularis) and the agents of Lyme disease and human granulocytic ehrlichiosis in a New York City park. Emerg Infect Dis. 1997;3:353–5. DOIPubMedGoogle Scholar
- Levin LM, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–8. DOIPubMedGoogle Scholar
- Bakken JS, Krueth J, Lund T, Malkovitch D, Asanovich K, Dumler JS. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin Infect Dis. 1996;23:198.PubMedGoogle Scholar
- Horowitz HW, Kilchevsky E, Haber S, Aguero-Rosenfeld M, Kranwinkel R, James EK, Perinatal transmission of the agent of human granulocytic ehrlichiosis. N Engl J Med. 1998;339:375–8. DOIPubMedGoogle Scholar
- Richter PJ, Kimsey RB, Madigan JE, Barlough JE, Dumler JS, Brooks DL. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J Med Entomol. 1996;33:1–5.PubMedGoogle Scholar
- Burkot TR, Maupin GO, Schneider BS, Denatale C, Happ CM, Rutherford JS, Use of a sentinel host system to study the questing behavior of Ixodes spinipalpis and its role in the transmission of Borrelia bissettii, human granulocytic ehrlichiosis, and Babesia microti. Am J Trop Med Hyg. 2001;65:293–9.PubMedGoogle Scholar
- Cinco M, Padovan D, Murgia R, Maroli M, Frusteri L, Heldtander M, Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J Clin Microbiol. 1997;35:3365–6.PubMedGoogle Scholar
- Guy E, Tasker S, Joynson DH. Detection of the agent of human granulocytic ehrlichiosis (HGE) in UK ticks using polymerase chain reaction. Epidemiol Infect. 1998;121:681–3. DOIPubMedGoogle Scholar
- Pusterla N, Leutenegger CM, Huder JB, Weber R, Braun U, Lutz H. Evidence of the human granulocytic ehrlichiosis agent in Ixodes ricinus ticks in Switzerland. J Clin Microbiol. 1999;37:1332–4.PubMedGoogle Scholar
- Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.PubMedGoogle Scholar
- Oteo JA, Gil H, Barral M, Pérez A, Jimenez S, Blanco JR, Presence of granulocytic ehrlichia in ticks and serological evidence of human infection in La Rioja, Spain. Epidemiol Infect. 2001;127:353–8. DOIPubMedGoogle Scholar
- Ogden NH, Bown K, Horrocks BK, Woldehiwet Z, Bennett M. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med Vet Entomol. 1998;12:423–9. DOIPubMedGoogle Scholar
- Alekseev AN, Dubinina HV, Van De Pol I, Schouls LM. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J Clin Microbiol. 2001;39:2237–42. DOIPubMedGoogle Scholar
- Cordas T, Aeschlimann A, Morel PC. Étude morphologique des ixodidae s. str. (Schulze, 1937) de Suisse au microscope électronique à balayage. Acaralogia. 1993;34:21–46.
- Dias JA. As carraças (Acari-Ixodoidea) da Península Ibérica. Algumas considerações sobre a sua biogeografia e relacionamento com a ixodofauna afropaleártica e afrotropical. Estudos. Ensaios e Documentos. 1994;158:1–163.
- Caspersen K, Park J-H, Patil S, Dumler JS. Genetic variability and stability of Anaplasma phagocytophila msp2 (p44). Infect Immun. 2002;70:1230–4. DOIPubMedGoogle Scholar
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–82. DOIGoogle Scholar
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8.PubMedGoogle Scholar
- Scorpio DG, Caspersen K, Ogata H, Park J, Dumler JS. Restricted changes in major surface protein-2 (msp2) transcription after prolonged in vitro passage of Anaplasma phagocytophilum. BioMedCentral Microbiology. 2004;4:1.PubMedGoogle Scholar
- Casey AN, Birtles RJ, Radford AD, Bown KJ, French NP, Woldehiwet Z, Groupings of highly similar major surface protein (p44)-encoding paralogues: a potential index of genetic diversity amongst isolates of Anaplasma phagocytophilum. Microbiology. 2004;150:727–34. DOIPubMedGoogle Scholar
- Parola P, Beati L, Cambon M, Brouqui P, Raoult D. Ehrlichial DNA amplified from Ixodes ricinus (Acari: Ixodidae) in France. J Med Entomol. 1998;35:180–3.PubMedGoogle Scholar
- Zeidner NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in northern Colorado. J Infect Dis. 2000;12:616–9. DOIPubMedGoogle Scholar
- Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. 2003;9:63–70.PubMedGoogle Scholar
- Goethert HK, Telford SR III. Enzootic transmission of the agent of human granulocytic ehrlichiosis among cottontail rabbits. Am J Trop Med Hyg. 2003;68:633–7.PubMedGoogle Scholar
- Holden K, Boothby JT, Anand S, Massung RF. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J Med Entomol. 2003;40:534–9. DOIPubMedGoogle Scholar
- Sixl W, Petrovec M, Marth E, Wust G, Stunzner D, Schweiger R, Investigation of Anaplasma phagocytophila infections in Ixodes ricinus and Dermacentor reticulatus ticks in Austria. Ann N Y Acad Sci. 2003;990:94–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 9—September 2004
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ana Sofia Santos, Centro de Estudos de Vectores e Doenças Infecciosas, Instituto Nacional de Saúde Dr. Ricardo Jorge, Avenida da Liberdade 5, 2965-575 Águas de Moura, Portugal; fax: +351-265-912568
Top